 |
 |
- Search
| Arch Aesthetic Plast Surg > Volume 25(3); 2019 > Article |
|
Abstract
Background
Serial volumetric changes of reconstructed breasts have not been studied in detail. In this study, we analyzed serial volumetric changes of reconstructed and contralateral normal breasts during long-term follow-up, with a focus on the effect of various adjuvant therapies.
Methods
Among all patients who underwent immediate breast reconstruction with a unilateral pedicled transverse rectus abdominis musculocutaneous (p-TRAM) flap, 42 patients with valid data from ≥3 postoperative positron emission tomography-computed tomography (PET-CT) scans were included. The volumes of the reconstructed and normal breasts were measured, and the ratio of flap volume to that of the contralateral breast was calculated. Serial changes in volume and the volume ratio were described, and the effects of chemotherapy, radiation therapy, and hormone therapy on volumetric changes were analyzed.
Results
The mean interval between the initial reconstruction and each PET-CT scan was 16.5, 30, and 51 months respectively. Thirty-five, 36, and 10 patients received chemotherapy, hormone therapy, and radiation therapy, respectively. The flap volume at each measurement was 531.0, 539.6, and 538.0 cm3, and the contralateral breast volume was 472.8, 486.4, and 500.8 cm3, respectively. The volume ratio decreased from 115.1% to 113.4%, and finally to 109.6% (P=0.02). Adjuvant therapies showed no significant effects.
Conclusions
We demonstrated that the p-TRAM flap maintained its volume over a long-term follow up, while the volume of the contralateral native breast slowly increased. Moreover, adjuvant breast cancer therapies had no statistically significant effects on the volume of the reconstructed p-TRAM flaps or the contralateral native breasts.
With advances in the knowledge and techniques of autologous breast reconstruction, complete flap survival has become a routine achievement, and perfecting the reconstruction in terms of aesthetics and patient satisfaction has emerged as an important issue. Volume retention of successfully transferred flap tissue in breast reconstruction procedures has not received much scientific attention, although some overcorrection is generally recommended, especially when adjuvant radiation is expected [1]. In contrast to free fat injection, for which the survival rate has been an important topic since its emergence [2,3], relatively little data have been published on the volume retention of transferred flaps in breast reconstruction procedures [4].
In contrast, volumetric changes of transferred flaps have been repeatedly investigated in the field of head and neck reconstruction because the volume retention of the flap is known to have significant functional effects [5-8]. Those results are complicated, because the patients in those studies usually suffered from severe oncologic conditions, and their oncologic state, eating function, and the volume of the flap were all mutually related. However, to the best of our knowledge, there is no universal agreement or available data on the volumetric changes of reconstructed and contralateral native breasts over the course of long-term follow-up. Therefore, we aimed to document serial volumetric changes of pedicled transverse rectus abdominis musculocutaneous (p-TRAM) flaps and the contralateral native breasts during long-term follow-up, with a focus on the possible effects of postoperative therapies such as chemotherapy, hormone therapy, and radiation therapy.
A database review for patients treated between April 2003 and April 2009 identified 400 consecutive patients who underwent unilateral immediate reconstruction using a pedicled TRAM flap. Among these, the patients with valid data from three or more postoperative positron emission tomography-computed tomography (PET-CT) scans were enrolled in the study protocol. All PET-CT scans were taken for oncologic purposes. The exclusion criteria were as follows: patients who underwent any contralateral procedures during the primary procedure or follow-up period, patients who underwent any secondary mound correction of the reconstructed breast during the follow-up period, patients who underwent any secondary therapies due to recurrence or metastasis after completion of the primary adjuvant therapies, and patients who were pregnant and/or gave birth to a child during the follow-up period. The study protocol was approved by the Institutional Review Board of Asan Medical Center. The informed consent was waived.
Patients were injected with 370–555 MBq (0.2 mCi/kg) of fludeoxyglucose and rested in a sitting or supine position for 60 minutes prior to scanning. Patients were positioned supine in the scanner with their arms above their heads. PET-CT scans were performed using Discovery STE (GE Healthcare, Chicago, IL, USA), Biograph TruePoint 16 (Siemens, Erlangen, Germany), and Biograph TruePoint 40 (Siemens) scanners, acquiring a total of five to six bed positions for 2–3 minutes per bed position. Calibration of each scanner against the dose calibrators and well counters was routinely performed. The measured standardized uptake values for the phantom were within the acceptable range of 90%–110%.
From the PET-CT data, the volume of the flap and the contralateral breast was calculated using the PetaVision picture archiving communication system software version 2.1 (Asan Medical Center, Seoul, Korea). Briefly, consecutive axial images were manually traced from the posterior margin, defined as the anterior surface of the pectoralis muscle fascia, to the anterior margin, defined as the outer surface of the skin. The measurements ranged vertically from the clavicle to the lower end of the breast mound, and horizontally from the lateral edge of the sternum to the lateral margin of the breast mound. The boundaries of the flap and the breast were manually traced throughout the entire measurement range and the volume was automatically calculated by integrating slice thickness (3 mm) over each polygonal area using the PetaVision software. Each measurement was repeated three times by the same person, who was blind to the study design, and the average was calculated for statistical analyses.
Each patient completed at least three postoperative PET-CT examinations at different time intervals from the reconstruction during the follow-up period, and the first three sets of measurements were evaluated. The average volume of the flap and the breast and the volume ratio of the flap to the contralateral breast at each examination were used to document serial changes. The effect of various adjuvant therapies, including chemotherapy, hormone therapy, and radiation therapy, was analyzed by a mixed analysis of variance model, using SPSS for Windows version 21.0 (IBM Corp., Armonk, NY, USA). P-values of less than 0.05 were considered to indicate statistical significance.
A total of 42 patients were included in the analysis. The average age of the patients at the time of reconstruction was 43.0 years (range, 28–64 years), and their mean body mass index (BMI) at the time of the operation was 22.15 kg/m2 (range, 17.6–27.5 kg/m2). The first three PET-CT scans were taken at 16.5, 30, and 51 months postoperatively on average. The mean age of the patients at each examination was 44.4, 45.5, and 47.3 years, respectively, and the mean BMI at each examination was 22.30, 22.28, and 22.46 kg/m2, respectively. The average BMI did not change significantly throughout the follow-up period.
The average volume of the flap at each examination was 531.0, 539.6, and 537.8 cm3, and the average volume of the contralateral breast was 472.8, 486.4, and 500.8 cm3, respectively (Fig. 1). The flap was always larger than the contralateral breast throughout the entire follow-up period, but the differences were not significant (P=0.09, P=0.10, and P=0.31, respectively). The volume ratio of the flap to the contralateral breast was used to better demonstrate trends in serial changes. The volume ratio, expressed as a percentage, was 115.1%, 113.4%, and 109.6%, respectively, showing a decrease over time (Fig. 2). The volume ratio was significantly lower at the third examination than at the first examination (P=0.02).
Among the 42 patients, 35 received chemotherapy. The overall trend in volume changes in the patients who received chemotherapy and those who did not is shown in Fig. 3. The seven patients who did not receive chemotherapy tended to have smaller flaps and breasts. Serial changes of the volume ratio and BMI ratio (current BMI to preoperative BMI) are shown in Fig. 4; there were no significant differences (P=0.10 and P=0.23, respectively).
Thirty-six patients underwent hormone therapy. The six patients who did not also had smaller breasts and flaps than those who received antiestrogen therapy (Fig. 5). However, the volume ratio was not significantly different between those two groups of patients (P=0.10) (Fig. 6).
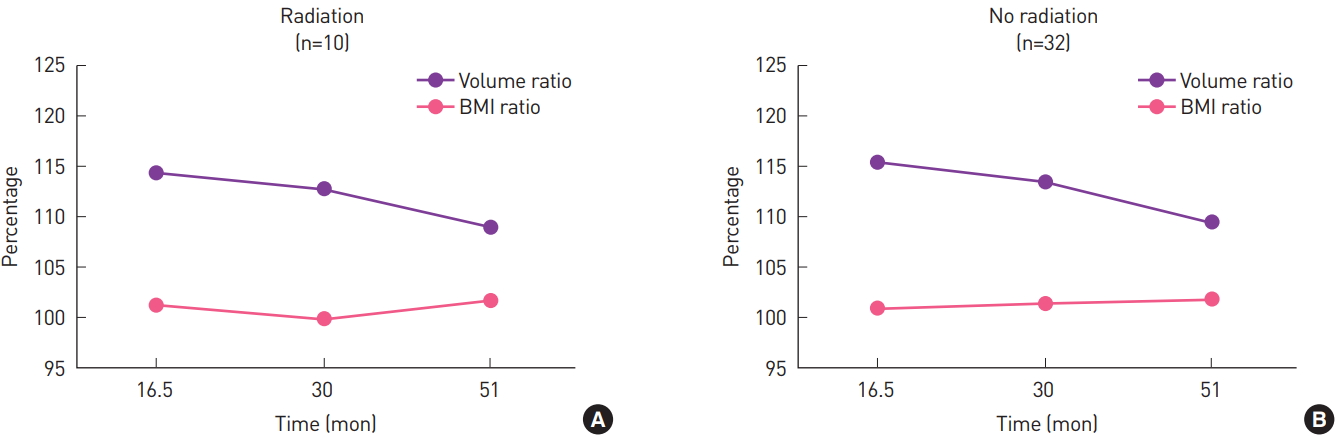
Ten patients underwent adjuvant radiation therapy. The average volume of the flaps in the patients who received radiation therapy continued to decrease over time (Fig. 7). However, the final flap volume was not significantly smaller than the first measurement in those patients (P=0.16). The volume ratio was not significantly different between those two groups of patients (P=0.08) (Fig. 8).
The volume of the reconstructed breast is an aesthetically important issue, similarly to how the volume of the reconstructed tongue is a functionally important issue in head and neck reconstruction, and reconstructive surgeons expect both the physical properties and the volume of a flap to be maintained after transfer. However, previous studies in the field of head and neck reconstruction have reported conflicting data [5-8].
Measuring the volume of a flap after reconstruction is not very simple, since the water displacement technique using Archimedes’ principle is not an option. Volume measurements utilizing image modalities are generally simpler and less time-consuming than direct measurements such as casting or measuring devices [9-14]. We manually traced two-dimensional PET-CT images taken for oncologic follow-up purposes and used the average of three measurements. The inter-measurement differences were usually less than 5% (data not shown). Volume measurements using three-dimensional images obtained by either volume rendering of two-dimensional images or surface scanning [9-11] could be even simpler, but deviations might be caused by position changes or respiration [15].
Chemotherapy for breast cancer has been generally known to increase weight and body fat. Nissen et al. [16] reported that weight gain was dependent on patients’ original body weight, and Sheean et al. [17] found that body weight did not consistently increase in women treated for breast cancer. However, the direct effect of chemotherapy on the flap and the contralateral breast during the treatment period was difficult to determine based on our study findings, since the first PET-CT examination was always taken well after the completion of chemotherapy. In our study, only seven patients did not receive chemotherapy, and they happened to have smaller breasts and flaps. However, the volume of the flap was maintained in those patients, with changes of less than 5% in both groups, and there were no significant differences in the volume ratio or BMI over time between the two groups.
According to Sheean et al. [17], the use of tamoxifen had a clear negative impact on adiposity. It is also known to increase visceral fat [18] and the percentage of body fat [19], and to expand abdominal adipose depots [20]. In our study, only six patients did not receive hormone therapy, which is a similar number to that of patients who did not receive chemotherapy. These small numbers may have been a result of the specific patient selection criteria, which required at least three sets of PET-CT data for enrollment. Nonetheless, the measurements revealed that patients who did not undergo hormone therapy also tended to have smaller flaps and breasts. Those patients also had the most unstable BMI ratio, with changes from 101.6% to 99.4% and finally to 103.4% at each examination, whereas the BMI ratio of the patients who underwent hormone therapy was 100.7%, 101.1%, and 101.5% at each examination, respectively. These results surprisingly indicate that the flap volume and the BMI of the patients who received adjuvant therapies for breast cancer were fairly well-maintained throughout the treatment period. The small number of the patients who did not have receive chemotherapy or hormone therapy might limit the generalizability of our findings, but there were no significant differences in the volume ratio or BMI over time between patients who did receive adjuvant therapies and those who did not.
The effects of radiation therapy on flaps have been investigated, and the earlier recommendation was against immediate reconstruction when adjuvant radiation is anticipated [21]. Recent systematic reviews, however, showed that the results of post-reconstruction irradiation are acceptable, especially in cases of autologous reconstruction [22-24], and Chatterjee et al. [25] and Clarke-Pearson et al. [26] also confirmed that post-reconstruction irradiation had no significant negative impact on the volume of deep inferior epigastric artery perforator free flaps. However, the volume of the flaps in the 10 patients who received radiation therapy in our study did decrease over time, although the differences were not significant. An increase in the contralateral breast over time was not evident in the patients who underwent radiation therapy, which could not be explained by the direct effects of irradiation. The flap volume ratio was comparable between patients who received radiation therapy and those who did not. Our study findings indicate that radiation caused no significant long-term decrease in flap volume. However, the possibility of long-term negative effects of radiation on the volume and cosmetics of the flap cannot be completely excluded, as Schaverien et al. [27] found that the proportion of patients who required revisional surgery was higher for immediate reconstruction with adjuvant radiotherapy than for delayed reconstruction. Therefore, we still recommend some overcorrection in volume when adjuvant radiation therapy is considered to be highly likely.
The small number of patients enrolled in our study greatly limits our analytic understanding of the independent postoperative effect of each adjuvant therapy. The overlapping therapies were not analyzed separately in order to preserve statistical power, since the number of patients allotted to each separate group would decrease significantly when divided into multiple non-overlapping groups. The sample size may have been limited because we only included patients with multiple PET-CT scans, which may also have been a source of bias in this study. Nonetheless, our integrative analysis of the effects of chemotherapy, hormone therapy, and radiation therapy seems to have yielded reasonable results, because only radiation therapy (although not to a significant extent) was demonstrated to have an effect on flap volume, which coincides with previous findings in the literature.
Interestingly, the volume of the contralateral native breast increased slowly over time, while the volume of the reconstructed breast remained relatively stable. A gradual increase of the native breast volume has also been reported in the literature [28,29]. Possible long-term discrepancies in the behavior of the flap and the contralateral breast should be explained to patients as part of preoperative counseling.
In contrast, flap volume loss in head and neck reconstruction has been almost universally observed. Fujioka et al. [5] reported that the volume of free flaps decreased from 15.9% (non-irradiated) to 20.9% (irradiated) due to fatty tissue atrophy over a period of 6–9 months after surgery. Oashi et al. [30] reported that vascularized fat flaps in rats lost 44% of their weight after transplantation, mainly because of apoptosis of adipocytes. However, Higgins et al. [8] reported a volume loss of only about 8% in anterolateral thigh free flaps following radiation, and Sakamoto et al. [6] observed that flap volume loss was primarily due to muscle atrophy, and that the thickness of the fatty tissue was relatively well maintained. Therefore, the volume loss of adipocutaneous flaps used for head and neck reconstruction does not seem to be essential or intrinsic, but instead appears to arise from the combined effects of impaired function, malnutrition, and radiation.
One of the limitations of our study was the relatively small number of patients in certain subgroups, especially patients who did not undergo chemotherapy or hormone therapy. Further studies with a larger number of patients and more frequent volume measurements during adjuvant therapy are required to definitively address the effect of each therapy on flap volume and body fat. The other main limitation was that intraoperative or immediate postoperative volume data were lacking. Some overcorrection could be assumed from the results we demonstrated. The early postoperative changes of the flaps were beyond the scope of this study, as the first measurements were taken at an average of 16.5 months post-reconstruction, when the initial postoperative changes and the acute effects of radiation had all subsided.
We showed that TRAM flaps maintained their volume during a long-term follow up, that the volume of the contralateral native breast slowly increased over time, and that various adjuvant therapies did not result in any statistically significant differences in the volume ratio of the flap to the contralateral breast.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2017-1034) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived.
Fig. 1.
Volume of the flap and the contralateral breast (cm3) at each measurement. Flap volume was relatively stable, while the volume of the contralateral native breast kept increasing.
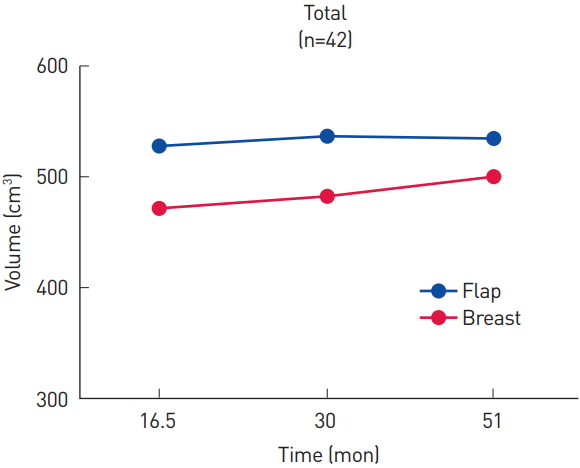
Fig. 2.
Overall volume ratio and the BMI ratio (BMI at the time of the PET-CT examination to the preoperative BMI). The volume ratio was significantly lower at the third measurement than at the first measurement. BMI, body mass index; PET-CT, positron emission tomography-computed tomography. a)P< 0.05.
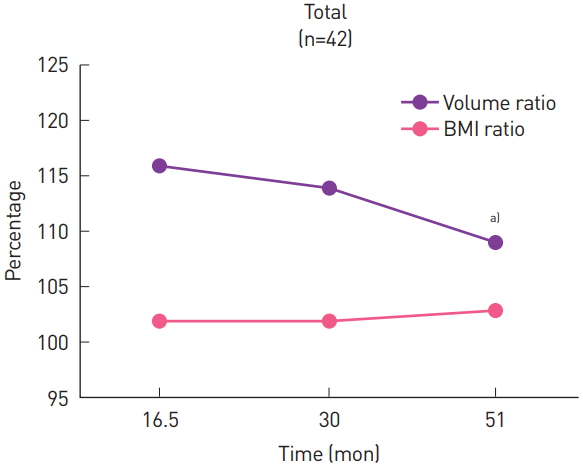
Fig. 3.
(A, B) Volume changes of the flap and the contralateral breast in patients who received chemotherapy and those who did not. The volumes of the flap and the breast tended to be smaller in patients who did not undergo chemotherapy.
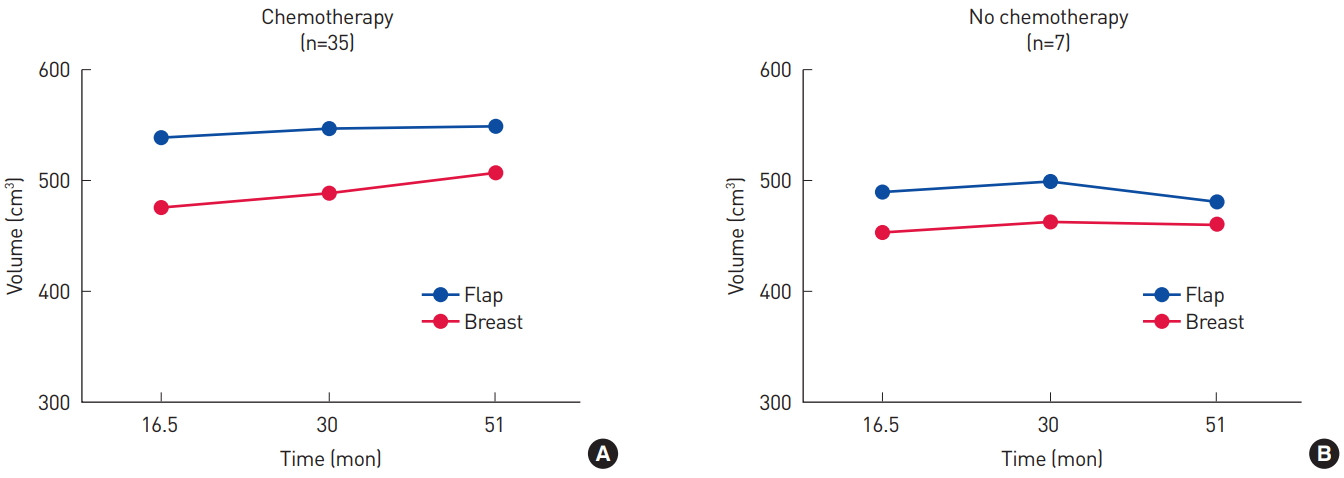
Fig. 4.
(A, B) Volume ratio and the BMI ratio (BMI at the time of the PET-CT examination to the preoperative BMI) of patients who received chemotherapy and those who did not. There were no statistically significant differences. BMI, body mass index; PET-CT, positron emission tomography-computed tomography.
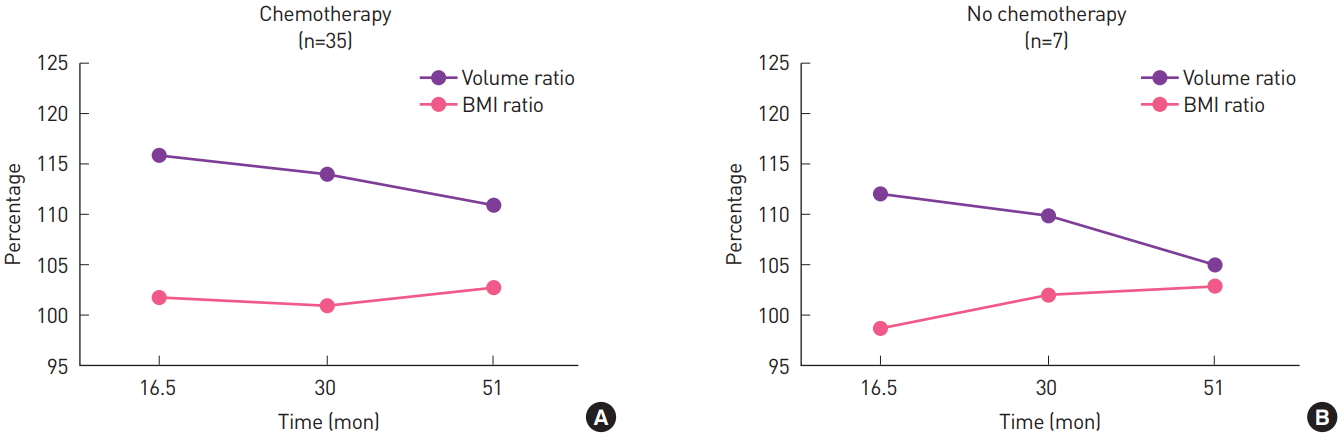
Fig. 5.
(A, B) Volume changes of the flap and the contralateral breast in patients who received hormone therapy and those who did not. The volumes of the flap and the breast tended to be smaller in those who did not undergo hormone therapy.
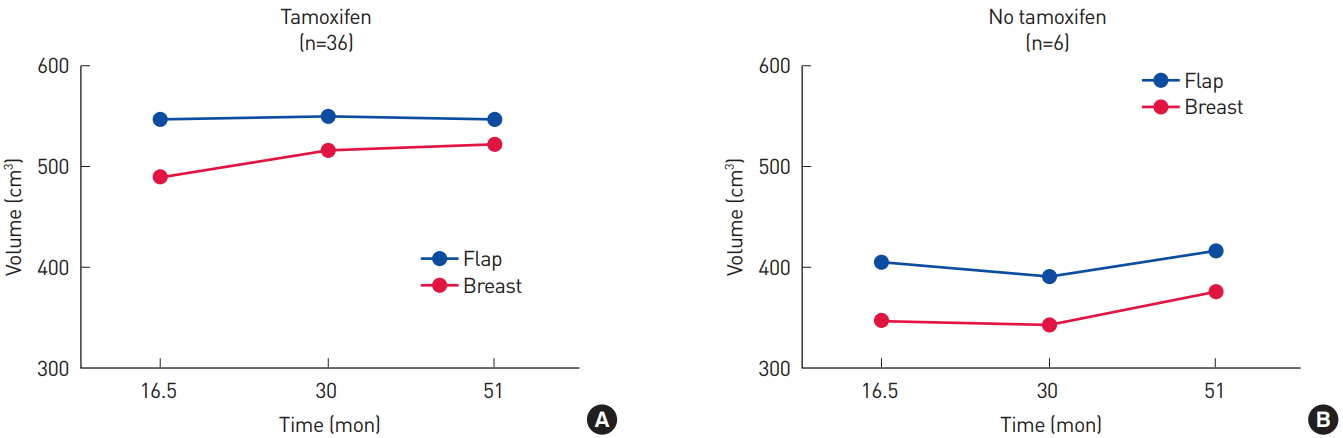
Fig. 6.
(A, B) Volume ratio and the BMI ratio (BMI at the time of the PET-CT examination to the preoperative BMI) of patients who received hormone therapy and those who did not. There were no statistically significant differences. BMI, body mass index; PET-CT, positron emission tomography-computed tomography.

Fig. 7.
(A, B) Volume changes of the flap and the contralateral breast in patients who received adjuvant radiation and those who did not. The volume of the flap slowly decreased over time in the patients who received radiation, but not to a statistically significant extent.

Fig. 8.
(A, B) Volume ratio and the BMI ratio (BMI at the time of PET-CT examination to the preoperative BMI) of patients who received adjuvant radiation and those who did not. There were no statistically significant differences. BMI, body mass index; PET-CT, positron emission tomographycomputed tomography.
REFERENCES
1. Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Part II. Breast reconstruction after total mastectomy. Plast Reconstr Surg 2009;123:794-805.


2. Herold C, Ueberreiter K, Busche MN, et al. Autologous fat transplantation: volumetric tools for estimation of volume survival. A systematic review. Aesthetic Plast Surg 2013;37:380-7.



3. Choi M, Small K, Levovitz C, et al. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg 2013;131:185-91.


4. Park SU, Shim JS. Assessment of breast volume change after transverse rectus abdominis myocutaneous flap. Arch Plast Surg 2012;39:631-5.




5. Fujioka M, Masuda K, Imamura Y. Fatty tissue atrophy of free flap used for head and neck reconstruction. Microsurgery 2011;31:32-5.


6. Sakamoto Y, Takahara T, Ota Y, et al. MRI analysis of chronological changes in free-flap volume in head and neck reconstruction by volumetry. Tokai J Exp Clin Med 2014;39:44-50.

7. Cho KJ, Joo YH, Sun DI, et al. Perioperative clinical factors affecting volume changes of reconstructed flaps in head and neck cancer patients: free versus regional flaps. Eur Arch Otorhinolaryngol 2011;268:1061-5.



8. Higgins KM, Erovic BM, Ravi A, et al. Volumetric changes of the anterolateral thigh free flap following adjuvant radiotherapy in total parotidectomy reconstruction. Laryngoscope 2012;122:767-72.


9. Hoeffelin H, Jacquemin D, Defaweux V, et al. A methodological evaluation of volumetric measurement techniques including three-dimensional imaging in breast surgery. Biomed Res Int 2014;2014:573249.




10. Fujii T, Yamaguchi S, Yajima R, et al. Accurate assessment of breast volume by computed tomography using three-dimensional imaging device. Am Surg 2012;78:933-5.


11. Kovacs L, Eder M, Hollweck R, et al. Comparison between breast volume measurement using 3D surface imaging and classical techniques. Breast 2007;16:137-45.


12. Kayar R, Civelek S, Cobanoglu M, et al. Five methods of breast volume measurement: a comparative study of measurements of specimen volume in 30 mastectomy cases. Breast Cancer (Auckl) 2011;5:43-52.



13. Kim H, Mun GH, Wiraatmadja ES, et al. Preoperative magnetic resonance imaging-based breast volumetry for immediate breast reconstruction. Aesthetic Plast Surg 2015;39:369-76.



14. Caruso MK, Guillot TS, Nguyen T, et al. The cost effectiveness of three different measures of breast volume. Aesthetic Plast Surg 2006;30:16-20.



15. Liu C, Ji K, Sun J, et al. Does respiration influence breast volumetric change measurement with the three-dimensional scanning technique? Aesthetic Plast Surg 2014;38:115-9.



16. Nissen MJ, Shapiro A, Swenson KK. Changes in weight and body composition in women receiving chemotherapy for breast cancer. Clin Breast Cancer 2011;11:52-60.


17. Sheean PM, Hoskins K, Stolley M. Body composition changes in females treated for breast cancer: a review of the evidence. Breast Cancer Res Treat 2012;135:663-80.




18. Nguyen MC, Stewart RB, Banerji MA, et al. Relationships between tamoxifen use, liver fat and body fat distribution in women with breast cancer. Int J Obes Relat Metab Disord 2001;25:296-8.



19. Ali PA, al-Ghorabie FH, Evans CJ, et al. Body composition measurements using DXA and other techniques in tamoxifen-treated patients. Appl Radiat Isot 1998;49:643-5.


20. Shea KL, Gavin KM, Melanson EL, et al. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause 2015;22:1045-52.



21. Spear SL, Ducic I, Low M, et al. The effect of radiation on pedicled TRAM flap breast reconstruction: outcomes and implications. Plast Reconstr Surg 2005;115:84-95.


22. Berbers J, van Baardwijk A, Houben R, et al. ‘Reconstruction: before or after postmastectomy radiotherapy?’ A systematic review of the literature. Eur J Cancer 2014;50:2752-62.


23. Shah C, Kundu N, Arthur D, et al. Radiation therapy following postmastectomy reconstruction: a systematic review. Ann Surg Oncol 2013;20:1313-22.



24. Kelley BP, Ahmed R, Kidwell KM, et al. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Ann Surg Oncol 2014;21:1732-8.




25. Chatterjee JS, Lee A, Anderson W, et al. Effect of postoperative radiotherapy on autologous deep inferior epigastric perforator flap volume after immediate breast reconstruction. Br J Surg 2009;96:1135-40.


26. Clarke-Pearson EM, Chadha M, Dayan E, et al. Comparison of irradiated versus nonirradiated DIEP flaps in patients undergoing immediate bilateral DIEP reconstruction with unilateral postmastectomy radiation therapy (PMRT). Ann Plast Surg 2013;71:250-4.


27. Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice?: a systematic review of the literature. J Plast Reconstr Aesthet Surg 2013;66:1637-51.


28. Hammann-Kloss JS, Bick U, Fallenberg E, et al. Volumetric quantification of the effect of aging and hormone replacement therapy on breast composition from digital mammograms. Eur J Radiol 2014;83:1092-7.


-
METRICS

- Related articles in AAPS
-
Delivery technique for the pedicled transverse rectus abdominis myocutaneous flap2022 October;28(4)






