|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 27(3); 2021 > Article |
|
Abstract
Background
For the correction of small breasts with grade I ptosis, it is very challenging for plastic surgeons to obtain excellent aesthetic results by performing simultaneous breast augmentation and nipple-areolar complex (NAC) lifting. Previous research has introduced one-stage augmentation mastopexy, but most studies described using the periareolar approach. The current study proposes a technique for augmentation mastopexy using the inframammary fold approach for augmentation and the periareolar approach for mastopexy.
Methods
Twenty patients were enrolled, and surgery was performed on 40 breasts. A pocket was made with the inframammary fold approach and the dual-plane method; subsequently, a tear-drop shape implant was inserted using a funnel. We performed NAC lifting using the de-epithelialization and interlocking purse-string suture method through the periareolar approach.
Results
The mean distance from the mid-clavicular line to the nipple was 23.4 cm preoperatively, 19.6 cm at 7 days of follow-up, and 20.3 cm at 12 months of follow-up. Complications such as hematoma, infection, NAC necrosis, capsular contracture, and wound dehiscence were not reported.
Conclusions
We performed successful breast augmentation and mild ptosis correction. No specific complications were observed during 1 year of postoperative follow-up. Our method is a simple and fast method that enables surgeons to perform augmentation and mastopexy in one stage for breasts with grade I ptosis.
Beautifully shaped, ŌĆ£perkyŌĆØ breasts are a symbol of youth and sexuality, and the lack of this trait can cause insecurity and self-esteem issues for many women. Therefore, breast augmentation has been one of the most popular cosmetic surgical procedures for years. In the United States, over 285,000 breast augmentation procedures were performed in 2014, second only to liposuction [1]. However, if ptosis simultaneously occurs, augmentation alone does not produce the desired aesthetic result. In cases of grade I breast ptosis according to the Regnault classification, with a relatively appropriate amount of breast volume, nipple-areolar complex (NAC) lifting can be performed [2]. Therefore, if patients have breast ptosis and small breast volume, both breast augmentation and mastopexy are needed.
One-stage augmentation mastopexy is a contradictory procedure: augmentation stretches the skin envelope and breast tissue by increasing breast volume, whereas mastopexy diminishes the skin envelope and may also decrease breast volume. However, one-stage augmentation mastopexy has been performed with increasing frequency due to patientsŌĆÖ desire to enhance breast fullness and correct breast ptosis in a single surgical procedure [3]. One-stage augmentation mastopexy can be achieved through skin reduction with implant insertion.
Periareolar augmentation mastopexy involves annular resection of the periareolar skin, which is pulled around the NAC while reducing the surrounding soft tissue envelope if necessary [4,5]. This approach has the advantage of a less visible incision scar, but damage of the lactiferous duct can occur [6]. In addition, impaired breastfeeding and sensory loss of the NAC may take place, which are particular concerns for unmarried women [7].
In our clinic, before 2010, one-stage augmentation mastopexy was performed with the periareolar approach. However, hypertrophic scar and widening occurred at the incision site due to tension caused by augmentation, and lactiferous duct injury occurred in about one-third of cases. When performing postoperative correction, re-access via the existing incision site made the resolution process complicated. Using the periareolar approach, it was also difficult to secure the field of view during pocket dissection, and it took longer because more delicate techniques were required to minimize the risk of lactiferous duct injury and scars at the junction between the areola and the breast mound.
To solve these problems, we introduce our technique for breast augmentation using the inframammary fold approach with simultaneous mastopexy using the periareolar approach.
This study was performed from 2016 to 2018 and included 20 female patients (40 breasts). The Institutional Review Board of Soonchunhyang University Gumi Hospital (IRB No. 2020-12) approved our study. The ideal candidate for this procedure had grade I ptosis according to the Regnault classification with insufficient breast volume.
During the initial physical examination, we focused on assessing the degree of breast ptosis and the amount of skin excess of the breast. The distance from the mid-clavicular line to the nipple was measured preoperatively. Breast width was measured to select the appropriate implant. Considering patientsŌĆÖ desires, we limited implant projection to a one-stage procedure to minimize stress on the skin and soft tissue due to mastopexy.
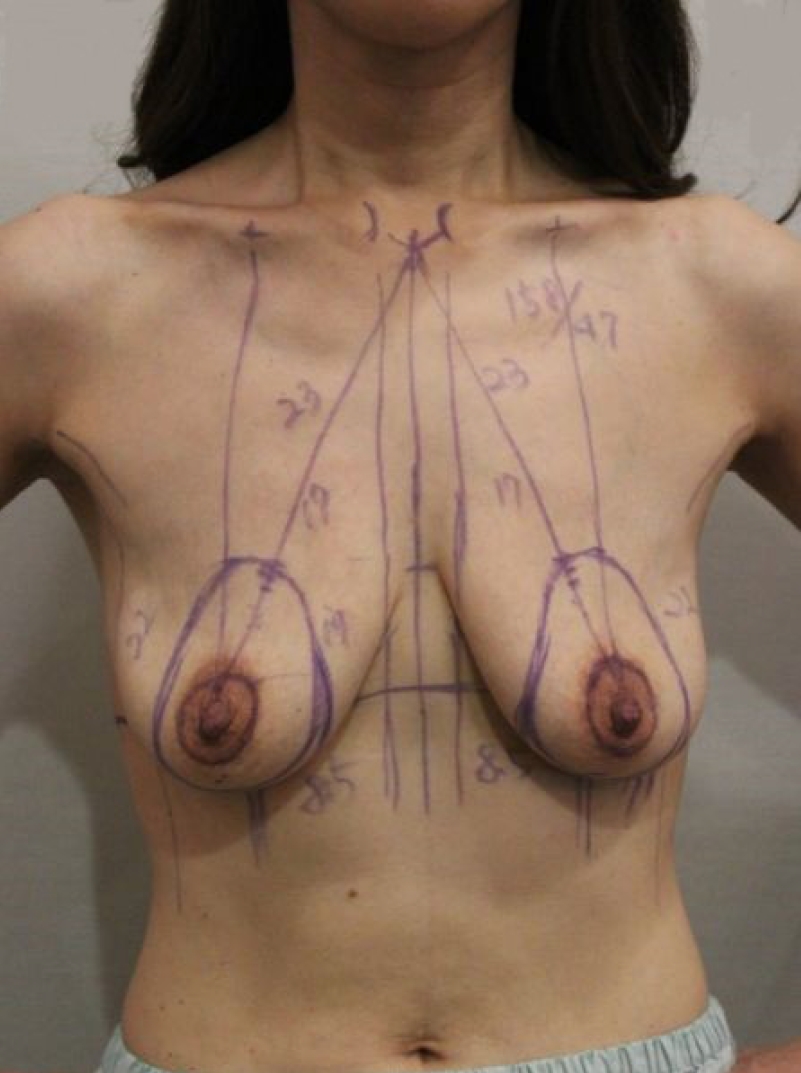
Preoperative marking was performed with the patient in the standing position (Fig. 1). The midline between the sternal notch and xiphoid process was marked, and the distance from the mid-clavicular area to the nipple was measured. Using bimanual palpation, the level of the inframammary crease was transposed to the anterior surface of one of the breasts. This portion corresponded to the superior border of the newly placed NAC.
The marking for the inframammary augmentation incision line was drawn at the inframammary fold and the marking for the periareolar mastopexy was drawn at the NAC. After breast implantation, the tissue is stretched in a more horizontal than vertical direction. Therefore, we drew a vertically long oval shape as the outer incision line marking and a 40-mm-diameter circle as the inner incision line marking for the new NAC margin. When we designed the oval shape, the vertical length was determined by a pinch measurement to calculate the excess skin and the horizontal length was determined as 80% of the vertical length.
Prophylactic antibiotics were administered 30 minutes before surgery. Both breasts, upper arms, and the abdomen were prepped and draped in the usual manner. We also applied a transparent barrier to the NAC before the surgical incision to minimize bacterial contamination.
First, local anesthesia with epinephrine was injected into the incision site and breast pocket for implant insertion. After incision through the inframammary fold approach, meticulous hemostasis was performed and the space into which the breast implant was to be inserted was dissected. For the implant pocket, the dual-plane method was used where the upper portion of the implant was located in the subpectoral space and the lower portion was located in the subglandular space. Dissection continued with electrocautery to identify the avascular loose areolar plane between the breast parenchyma and pectoralis major fascia. The location for splitting the pectoralis major muscle was individualized according to the patientŌĆÖs extent of lower pole parenchyma. In patients lacking lower pole parenchyma, the pectoralis major muscle was transversely split near the inframammary fold, so that only the lower part of the lower pole was placed in the subglandular layer. Instead, if the lower pole parenchyma was sufficient, the pectoralis major muscle was split at the NAC position so that more than half of the lower portion of the implant was positioned in the subglandular layer. Over dissection should be avoided in the medial and lateral directions to prevent implant malposition. After dissection, a sizer was used to determine the appropriate size of the breast implant and to determine whether sufficient dissection had been performed.
Once pocket dissection was complete and hemostasis had been achieved, the breast pockets were irrigated with antibiotic solution (cefazolin [1 g], ceftriaxone [1 g], and gentamycin [500 mg] in 500 mL of normal saline). Subsequently, we inserted the implant and set the operating table to 60┬░ to confirm the symmetry and laxity or tension of the skin and soft tissue. Surgery was performed with the no-touch technique by using a funnel when applying the sizer and implant (Mentor CPG silicone gel implant; Johnson & Johnson, New Brunswick, NJ, USA). The implant was only handled by the operating surgeon. No drain was inserted.
After finishing the suture of the inframammary fold incision site, we removed the transparent barrier applied to NAC and performed aseptic draping again. The breast skin was confirmed to be stretched due to the implantation, and it was stretched more in the horizontal direction, as expected preoperatively. Thus, the preoperative oval design marking was observed as a circular shape.
The initial periareolar marking and NAC margin incisions were made using a no. 15 blade scalpel, and an oval annular donut of periareolar skin was de-epithelialized with a no. 20 blade scalpel. Next, the periareolar skin was circumferentially undermined in the subcutaneous plane for approximation. During undermining, dissection should be carefully performed on the subcutaneous plane to prevent implant exposure.
After excision of the periareolar skin and undermining of the surrounding tissue, the NAC and outer margin of the incision were approximated using a stapler. We confirmed that the NAC was properly positioned, and then an interlocking purse-string suture was performed using a Gore-Tex suture on a CV-3 long straight needle. The NAC and periareolar skin at the 3-, 6-, 9-, and 12-oŌĆÖclock positions were marked before performing the interlocking purse-string suture, which provided eight reference markings for closure. The interlocking suture was performed at the 9- and 3-oŌĆÖclock positions on the right and left breasts, respectively, starting from the outer circle, using the Hammond method [8]. Finally, the periareolar incision site was closed with an interrupted subcutaneous suture by 4-0 Vicryl, and Dermabond was applied to the skin. A schematic illustration of the periareolar mastopexy is shown in Fig. 2.
Both the inframammary fold and periareolar incisions were dressed postoperatively using foam for protection. Patients underwent compression through a surgical bra to prevent hematoma in the implant pocket. All patients visited our clinic the next day after surgery, and were followed up once a week during the first month postoperatively. Scar care with silicone sheeting was initiated once the incision was completely healed. Thereafter, follow-up was performed every 3 months until 1 year.
Statistical analyses were conducted using SPSS statistical software version 27.0 (IBM Corp., Armonk, NY, USA). Quantitative variables were described using mean values. The Shapiro-Wilk test showed that the small dataset did not follow a normal distribution, so a nonparametric method was used for data analysis. A 95% confidence interval (95% CI) was calculated, and 5% significance level was considered, as reflected by a P-value <0.05. P-values were calculated using the Wilcoxon signed rank test for continuous variables.
We performed a retrospective review of all patients who underwent one-stage augmentation mastopexy by a single plastic surgeon. From January 2016 to June 2018, 20 women and 40 breasts were included. The mean age of the patients was 35 years (range, 21ŌĆō47 years) and the mean body mass index was 21.2 kg/m2. Teardrop-shaped silicone gel implants were used for all patients, with a mean implant volume of 288.5 cc (range, 230ŌĆō350 cc). PatientsŌĆÖ demographic data are shown in Table 1.
The mean distance from the mid-clavicular line to the nipple was 23.4 cm preoperatively, 19.6 cm at 7 days of follow-up, and 20.3 cm at 12 months of follow-up. Statistically significant differences were found between the preoperative and the 7-day postoperative distances (P <0.001), and between the preoperative and 12-month postoperative distances (P<0.001). The difference in the distance from the mid-clavicular line to the nipple between 7 days postoperatively and 12 months of follow-up was minimal and statistically insignificant (Table 2).
There were no cases of reoperation. Although some patients complained about the inframammary fold incision scar after surgery, the incisional scars were not noticeable after follow-up for more than 6 months. However, if the patient was concerned about scars that were not significant enough for an evaluation by the surgeon, laser treatment or scar revision was performed. No capsular contracture was observed based on visual examination and palpation during follow-up. Other complications such as hematoma, seroma, infection, NAC necrosis, NAC sensory loss, and wound dehiscence were not reported. In addition, skin irregularity in the periareolar area, which could be caused by the interlocking purse-string suture, was not found at the 12-month follow-up. The photographic findings of two patients are presented in Figs. 3 and 4.
Fifty years have passed since simultaneous augmentation mastopexy was initially described in the literature by Gonzalez-Ulloa [9]. The combination of implant augmentation or auto-augmentation and mastopexy has been reported by many researchers [10-12]. Although various procedures have been introduced in the past, augmentation and mastopexy continue to be challenging tasks for plastic surgeons. Despite the advantages of reduced exposure to a second round of anesthesia, reduced expenses, and a shorter recovery time, the combined procedure has historically been considered one of the most difficult cosmetic breast surgeries and has become the source of numerous litigious claims from dissatisfied patients [13-15]. Breast augmentation may result in skin stretching because of the use of implants or autologous tissue to reinforce insufficient tissue, whereas mastopexy requires excessive skin reduction; hence the two procedures are contradictory. Nonetheless, patients often request one-stage augmentation mastopexy because of the cost and convenience of one-stage surgery. As a result, various procedures have been developed. In the course of mastopexy, several surgeons have reported the choice of the pedicle, NAC elevation method, and augmentation material [16-18]. However, most previous research has described performing augmentation and mastopexy simultaneously through a periareolar incision.
Implant insertion through the conventional periareolar approach has the advantage of allowing surgery to be performed through the same incision site. However, there is an opening in the periareolar area of the lactiferous duct, and the injury of the lactiferous duct cannot be avoided if the incision is made in this area. Implant insertion through a periareolar incision involves difficult dissection because of the narrow field of view. Instead, under direct visualization through the inframammary fold approach, lower pole expansion and implantation are easier and the operative time is reduced. Moreover, reducing tissue injury around the NAC makes it possible to minimize NAC sensory loss and duct damage, which is particularly important for unmarried women due to the impact of those complications on breastfeeding.
In this study, implants were inserted using the inframammary fold approach, which was intended as a way to perform a more aseptic procedure without damaging the lactiferous duct. In addition, the surgery was performed using the no-touch technique, wherein an implant was inserted using a funnel, and only the surgeon palpated the implant after performing a glove change. We both minimize implant exposure to the outside environment and avoid any contact between the implant and skin. Because of this process, postoperative bacterial contamination did not occur.
According to a previous study about the concurrent approach through the inframammary fold and periareolar area, there was a concern regarding the occurrence of animation deformity because the muscle was possibly manipulated to shape the submuscular pocket for implant insertion [19]. However, as described in the current study, using an inframammary fold incision without manipulation of the muscles has the advantages of a shorter operation time and a reduced risk of animation deformity.
Furthermore, during periareolar mastopexy, the dermis between the outer and inner incision sites was preserved. In this way, we aimed to reduce the possibility of nipple necrosis by preserving blood circulation around the NAC. Additionally, only performing de-epithelialization of periareolar tissue helped to minimize the risk of exposure of the inserted implant. Sufficient periareolar tissue undermining can reduce the widening of the NAC postoperatively, and interlocking purse-string sutures help distribute tension around the NAC, keeping the nipple shape in the correct position and sustaining the circular shape of the nipple during the follow-up period.
In our study, we evaluated the positional correction of the NAC after surgery by measuring the distance from the mid-clavicular line to the nipple. The mean distance from the mid-clavicular line to the nipple was 23.4 cm on average preoperatively, and the mean distance was 19.6 cm at 7 days postoperatively. After mastopexy, the NAC was newly placed 3.8 cm above its previous position. At 12 months of follow-up, this position had lowered by 7 mm on average compared to the immediately postoperative condition.
This method has disadvantages, such as being difficult to apply in cases of grade II or III ptosis according to the Regnault classification, because the periareolar mastopexy procedure described in our study may be insufficient to remove the redundant skin. Therefore, in patients with tubular-shaped breasts, the periareolar approach is more effective than our method. Another limitation is that the follow-up period was relatively short, for which reason it was not possible to analyze long-term results.
We performed successful breast augmentation and correction of grade I ptosis using the less invasive inframammary fold incision approach and NAC lifting through de-epithelialization without incision of the dermis. Compared to NAC lift and breast augmentation via periareolar incision at the same site, the operative time was shorter, and there was a low risk of bleeding and bacterial contamination. No specific complications were observed during the follow-up period, and the postoperative results were good. Therefore, the method presented herein is a simple and fast option for performing one-stage augmentation and mastopexy in breasts with grade I ptosis.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Soonchunhyang University Gumi Hospital (IRB No. 2020-12) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Fig.┬Ā2.
Illustration of the periareolar mastopexy procedure after augmentation. Red line: outer and inner circular incision lines; blue area: de-epithelization area; green line: after undermining of the surrounding tissue, an interlocking purse-string suture was done.
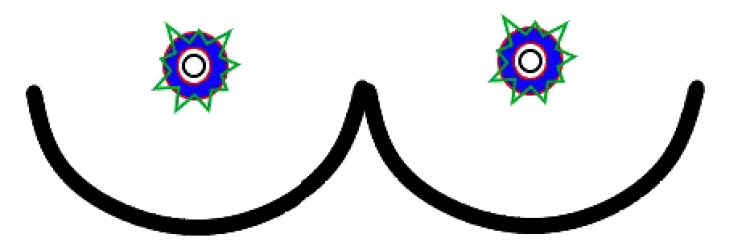
Fig.┬Ā3.
(A-C) Preoperative and (D-F) postoperative photographic findings of a 39-year-old woman taken 6 months after surgery.
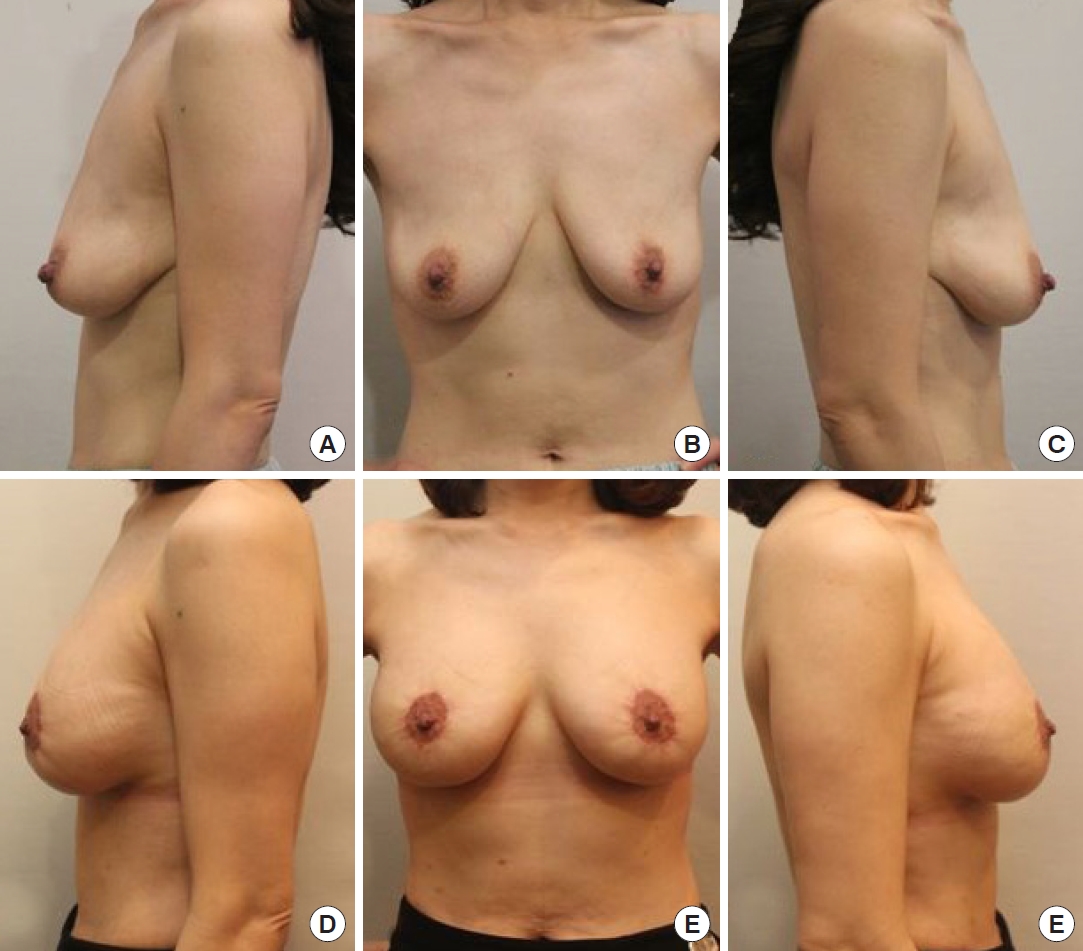
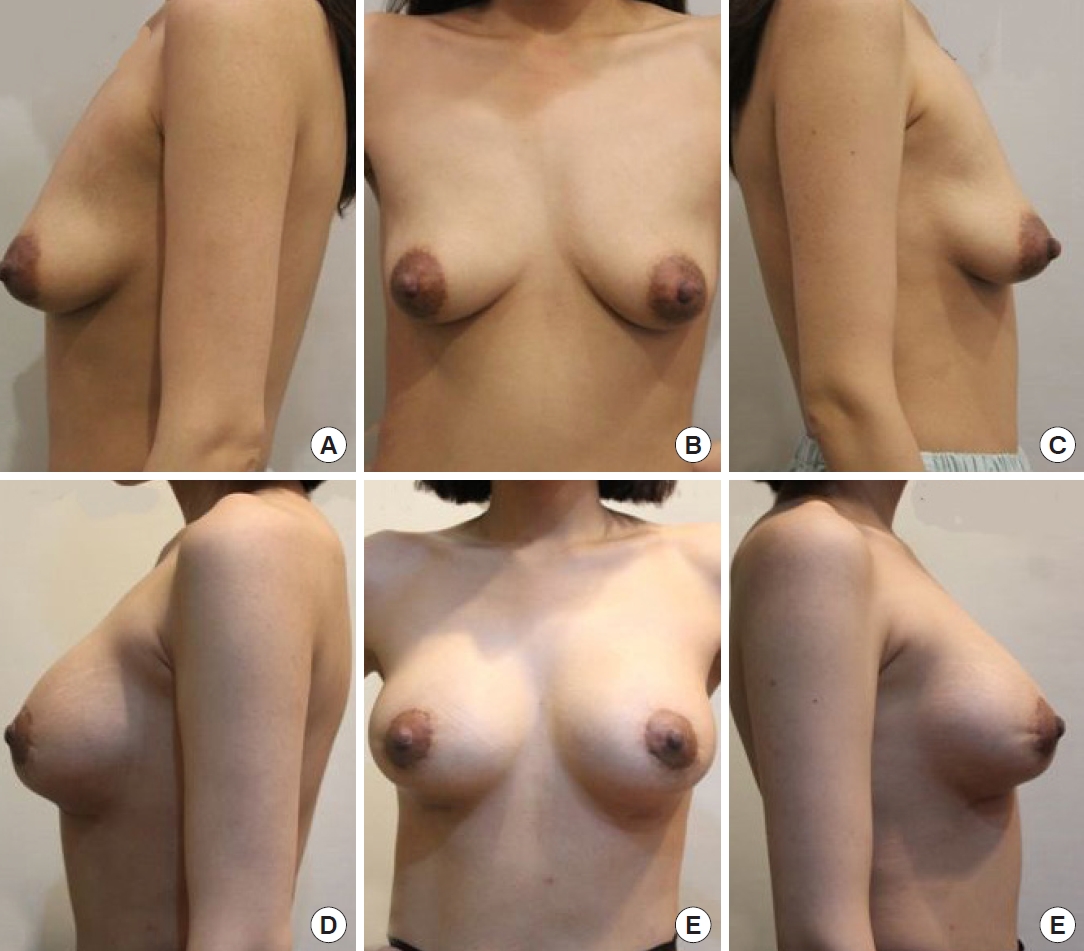
Fig.┬Ā4.
(A-C) Preoperative and (D-F) postoperative photographic findings of a 32-year-old woman taken 9 months after surgery.
Table┬Ā1.
Demographic data of patients
Table┬Ā2.
Distance from the mid-clavicular line to nipple (n=40)
| Variable | Preoperative | After 7 day | After 3 mo | After 6 mo | After 12 mo | P-valuea) | P-valueb) |
|---|---|---|---|---|---|---|---|
| Right (cm) | 23.55 (22.70ŌĆō24.07) | 19.25 (19.00ŌĆō20.00) | 19.75 (19.00ŌĆō20.00) | 20.00 (19.48ŌĆō20.35) | 20.25 (19.80ŌĆō20.50) | < 0.001 | < 0.001 |
| Left (cm) | 23.50 (22.95ŌĆō24.27) | 19.40 (19.00ŌĆō20.00) | 19.65 (19.35ŌĆō20.00) | 20.00 (19.50ŌĆō20.40) | 20.30 (19.95ŌĆō20.50) | < 0.001 | < 0.001 |
REFERENCES
1. American Society of Plastic Surgeons. Current procedural statistics [Internet] Garden Grove, CA: The Aesthetic Society; c2014 [cited 2021 Jan 20]. Available from: http://www.surgery.org
3. Stevens WG, Macias LH, Spring M, et al. One-stage augmentation mastopexy: a review of 1192 simultaneous breast augmentation and mastopexy procedures in 615 consecutive patients. Aesthet Surg J 2014;34:723-32.


4. Gruber RP, Jones HW Jr. The ŌĆ£donutŌĆØ mastopexy: indications and complications. Plast Reconstr Surg 1980;65:34-8.

5. Benelli L. A new periareolar mammaplasty: the ŌĆ£round blockŌĆØ technique. Aesthetic Plast Surg 1990;14:93-100.


6. Bachour Y, Verweij SP, Gibbs S, et al. The aetiopathogenesis of capsular contracture: a systematic review of the literature. J Plast Reconstr Aesthet Surg 2018;71:307-17.


7. Mofid MM, Klatsky SA, Singh NK, et al. Nipple-areola complex sensitivity after primary breast augmentation: a comparison of periareolar and inframammary incision approaches. Plast Reconstr Surg 2006;117:1694-8.


8. Righi B, Robotti E. Successfully exploiting two opposing forces: a rational explanation for the ŌĆ£interlocking sutureŌĆØ. Aesthetic Plast Surg 2011;35:177-83.


9. Gonzalez-Ulloa M. Correction of hypotrophy of the breast by means of exogenous material. Plast Reconstr Surg Transplant Bull 1960;25:15-26.


10. Owsley JQ Jr. Simultaneous mastopexy and augmentation for correction of the small, ptotic breast. Ann Plast Surg 1979;2:195-200.


12. Spear SL, Pelletiere CV, Menon N. One-stage augmentation combined with mastopexy: aesthetic results and patient satisfaction. Aesthetic Plast Surg 2004;28:259-67.


13. Gorney M. Ten yearsŌĆÖ experience in aesthetic surgery malpractice claims. Aesthet Surg J 2001;21:569-71.


14. Hoffman S. Some thoughts on augmentation/mastopexy and medical malpractice. Plast Reconstr Surg 2004;113:1892-3.


15. Paik AM, Mady LJ, Sood A, et al. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J 2014;34:79-86.

17. Honig JF, Frey HP, Hasse FM, et al. Autoaugmentation mastopexy with an inferior-based pedicle. Aesthetic Plast Surg 2009;33:302-7.