|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 28(2); 2022 > Article |
|
Abstract
Complications arising from breast augmentation procedures are broadly categorized as either surgery-related or prosthesis-related. Many reports have described complications associated with breast augmentation. However, to date, periareolar post-inflammatory hyperpigmentation (PIH) after breast augmentation has not been reported. Herein, we report a case of PIH after augmentation mammoplasty using a silicone implant through the periareolar approach. A 35-year-old woman, who underwent bilateral breast augmentation using a periareolar approach, presented with bilateral periareolar tissue changes, with dark brown, irregular macules appearing 6 weeks postoperatively. Based on clinical symptoms and histological examination, the lesion was diagnosed as PIH. Topical hydroquinone and retinoic acid were applied for 8 weeks after the pigmentation appeared. After 6 months of observation, the pigmentation faded. To summarize, we report a case of pigmentation around the bilateral nipples after periareolar breast augmentation along with a literature review.
Breast augmentation is one of the most common plastic surgery procedures performed worldwide. With the development of breast augmentation, incision placement has become an important element of the overall surgical plan. In this regard, the periareolar incision, first described by Jones and Tauras in 1973, is commonly used [1]. The most important advantage of the periareolar incision is that it provides a direct visual field to create a sufficient breast pocket and meticulous bleeding control. Meticulous bleeding control can lead to dry and blood-free pockets, thereby reducing the incidence of capsular contracture. Any type of breast implant can also be inserted through this incision, and immediate positioning of the inframammary fold can be comfortably performed. The scars between the pigmented skin of the areola and the lighter skin of the breast become unrecognizable over time. Although the periareolar approach has many advantages, it is also subject to several shortcomings. The use of the periareolar incision may be restricted according to the size of the areola, and during implant insertion, bacterial contamination around the duct is possible, leading to capsular contracture [2].
Many reports have described complications related to augmentation mammoplasty using silicone implants via the periareolar approach. However, pigmentation after breast augmentation using the periareolar approach has not yet been reported. In this study, we report a case of post-inflammatory hyperpigmentation (PIH) after augmentation mammoplasty using a silicone implant through a periareolar approach.
A 35-year-old woman underwent bilateral breast augmentation. Her skin type was Fitzpatrick skin phototype III. Preoperatively, her areolae were normal in color, but the periareolar zones of pigmentation were placed slightly more inferiorly than usual (Fig. 1). Augmentation mammoplasty was performed under general anesthesia. Using the periareolar incision, 350-mL microtextured cohesive gel silicone prostheses were placed bilaterally in the subpectoral pockets. The patient was discharged the following day. A follow-up appointment at 2 weeks showed a normal nipple-areolar complex with full recovery. Her progress was not otherwise specified. The patient did not notice any inflammatory changes on the breast skin. There were no inflammatory reactions requiring clinical intervention.
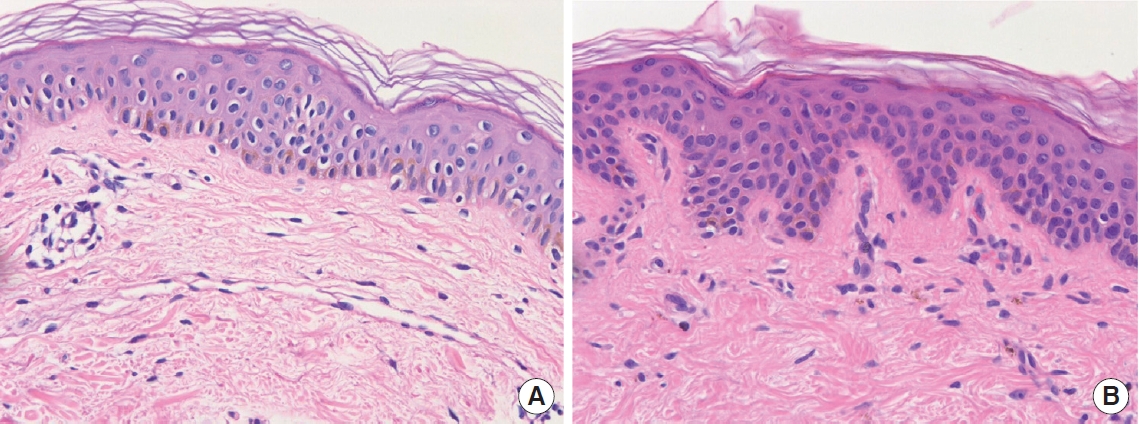
Over the next month, during her regular follow-up outpatient visits, the patient complained that her periareolar tissues were gradually becoming darker. A physical examination revealed bilateral asymmetric pigmentation or pigmentary incontinence in the subareolar regions. The progressive lesions showed skin hyperpigmentation without any erythematous changes or fluidic discharge. Within 6 weeks, brownish irregular macules under the periareolar incisions of the bilateral breasts became apparent. Her breasts otherwise had a natural look and feel, and a general examination revealed no similar lesions on her body. She had no regional lymphadenopathy, and her general condition was unremarkable. The clinical impression was that of post-inflammatory pigmentation (Fig. 2). Punch biopsies were taken from the centers of both hyperpigmented areolar lesions and normal sites of the right breast (Fig. 2). A few melanophages in the upper dermis were observed by hematoxylin and eosin staining (Fig. 3). Fontana-Masson silver staining showed a slightly elevated number of melanocytes in the epidermis of both areolar skin samples (Fig. 4). We diagnosed the patient with PIH. Topical hydroquinone (HQ) and retinoic acid were administered for 2 months. Three months after the operation, the pigmentation became faint but remained noticeable on the bilateral breasts (Fig. 5).
We encountered a case of PIH after breast augmentation using a periareolar incision, despite the absence of unusual findings in the postoperative course.
PIH is a disturbing sequela of inflammatory conditions such as endogenous cutaneous inflammation, external injury, or cutaneous procedures [3]. The causes of PIH can be divided into two broad categories: endogenous and exogenous factors. Inherited diseases, cutaneous diseases, and systemic diseases are endogenous factors that cause PIH. Exogenous factors capable of causing PIH include mechanical trauma, extremes of temperature, radiation, and phototoxic reactions [4]. Acne vulgaris and fractional ablative CO2 laser treatment are also common inflammatory skin disorders that can lead to the development of PIH [5]. However, there are few reports of PIH after a surgical incision, and we report one such case herein.
It is essential to differentiate between PIH and other causes of hyperpigmentation. Hyperpigmentation during pregnancy is a well-known physiologic, benign change due to increased levels of estrogen, progesterone, and melanocyte-stimulating hormone [6]. Pigmentated mammary Paget disease presents as an eczema-like lesion in the nipple and areolar skin, usually associated with intraductal mammary carcinoma. It is caused by non-neoplastic melanocytes of epidermal origin and should be distinguished from PIH [7]. The differential diagnosis includes primary pigmentary disorders such as lichen planus pigmentosus and erythema dyschromicum perstans [8]. The differential diagnosis of PIH is wide; however, a key clinical feature for identifying PIH is the history of a preceding inflammatory insult [9].
PIH can present in patients of all Fitzpatrick skin types; however, this type of hypermelanosis is more common in patients with a skin type of Fitzpatrick type III and higher [5]. Clinically, when melanin is confined to the epidermis, PIH appears as an asymptomatic, irregular, tanned macule or patch; however, blue-gray discoloration occurs when melanin is confined to the dermis [5]. Several pathways are involved in PIH following skin inflammation. First, the process results from an epidermal inflammatory response. The inflammatory response damages the cell membrane and causes the release and oxidation of arachidonic acid to prostaglandins or leukotrienes. Leukotrienes (LTC4, LTD4), prostaglandins (PGE2), and thromboxanes (TXB2) stimulate melanogenesis by upregulating tyrosinase and increasing melanocyte cell size and dendritic cell proliferation. LTC4 is the most potent factor for increasing tyrosinase activity and melanocyte growth [9]. Many other mediators, such as interleukin-1, interleukin-6, tumor necrosis factor-α, epidermal growth factor, and other keratinocyte-released chemical mediators (e.g., histamine) that stimulate melanocytes can also provoke their proliferation [5]. This leads to melanin synthesis and an increase in pigment transmission to the surrounding keratinocytes. Second, damage to the basal keratinocytes leads to dermal hypermelanosis. These degenerated keratinocytes contain a large amount of melanin and are eventually phagocytosed by macrophages in the upper dermis to form blue-gray discolorations [5]. Third, as part of the increase in dermal melanophages, the increase in intercellular edema prevents melanocytes from reaching keratinocytes, and the remaining melanin forms a melanosome complex later found within dermal melanophages [5]. The reaction to genetically innate hypermelanosis is also an important factor. Each person can be born with “weak” or “strong” melanocytes. If they have strong melanocytes, hyperpigmentation is likely to occur [10].
One of the most important treatment goals of PIH is to eliminate the cause of inflammation. Topical therapies are the most common and cost-effective treatment. HQ is the gold standard for the treatment of PIH. HQ inhibits tyrosinase and inhibits the conversion of dihydroxyphenylalanine to melanin, reducing the production of melanosomes [8]. Retinoid, a vitamin A analog, blocks the transcription of tyrosinase, which promotes melanin dispersion and removal to reduce epidermal pigments [11]. It reduces melanin uptake by increasing the turnover rate of cells and reducing the time of contact between melanocytes and keratinocytes [12]. Ever since Kligman et al. [13] first used a combination therapy of 5% HQ+0.1% tretinoin+0.1% dexamethasone in 1975, it has been known that combination therapy can have a significant impact on treatment outcomes and side effects. Photoprotection is an essential part of treatment. Sun avoidance and sun-protective measures are also beneficial for the management of PIH [8].
Although topical treatments remain the standard of care for treating PIH, lasers are increasingly being used as a treatment option. Kim et al. [14] reported that a Q-switched neodymium: yttrium-aluminum-garnet laser was effective in treating PIH. Lee et al. [15] found that a 1,064-nm picosecond-domain neodymium: yttrium-aluminum-garnet laser was effective in improving PIH. Chemical peels, such as salicylic acid or glycolic acid, effectively treat PIH by removing epidermal cells. Glycolic acid is a naturally occurring α-hydroxy acid that induces epidermolysis, disperses basal layer melanin, and increases dermal collagen synthesis; salicylic acid is a β-hydroxy acid that causes keratolysis by disrupting intercellular lipid linkages between epithelioid cells [3].
The patient in the case described herein developed PIH after breast augmentation using a periareolar incision and improved after 2 months of HQ and retinol combination therapy. We planned to use topical agents first and then further consider laser therapy if the topical agents were ineffective. PIH improved even though only topical agents were used, and no laser therapy was performed because the patient did not want further treatment. The patient may also have had melanocytes that were genetically more likely to cause hyperpigmentation. Because of the characteristics of a periareolar incision, it can be exposed to more bacteria on the ductal side during dissection [2], which may cause more epidermal inflammatory responses than other incisional methods, possibly inducing PIH.
In conclusion, this is the first case report to describe PIH after breast augmentation using a periareolar incision. Although many treatment options are available for PIH, PIH took months to resolve in this case, even with adequate therapy. Patient counseling regarding the natural course of PIH and its management plan is a helpful tool to assist the patient through the psychological stress of this condition.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Cheil General Hospital (CGH) & Women’s Healthcare Center (IRB No. 2018-48).
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Fig. 1.
Preoperative view. The areolae have a normal color and a slightly more inferiorly placed periareolar zone of pigmentation than usual, with Fitzpatrick skin phototype III.
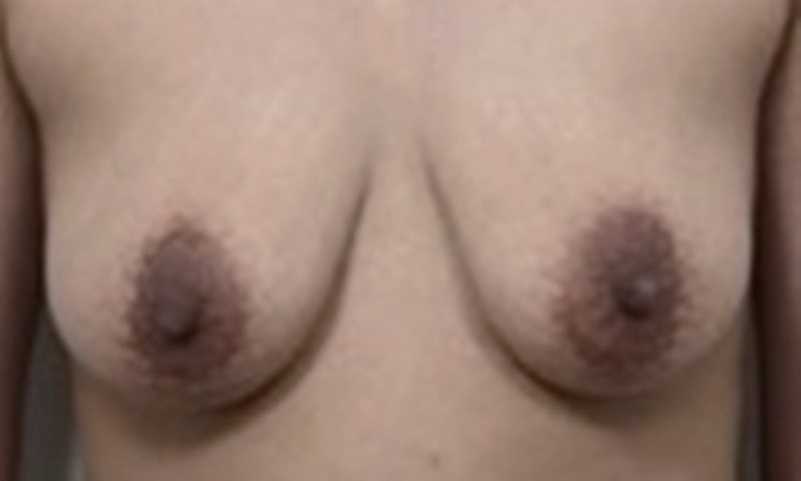
Fig. 2.
Pigmentation findings at 6 weeks after periareolar breast augmentation in a patient with Fitzpatrick skin phototype III. The red circles indicate a newly formed, irregular, tan-brown patch under the periareolar incision of the bilateral breasts. Punch biopsies were taken from the centers of both areolar hyperpigmented lesions (white arrows) and the normal site of the right breast (blue arrow). The clinical impression was post-inflammatory pigmentation.

Fig. 3.
Hematoxylin and eosin staining of normal skin and hyperpigmented lesions (×400). (A) Perilesional normal skin. (B) Hyperpigmented lesions. Histologic examination showing a few dermal macrophages in the upper layer of the dermis of the right breast.

REFERENCES
1. Jones FR, Tauras AP. A periareolar incision for augmentation mammaplasty. Plast Reconstr Surg 1973;51:641-4.


3. Callender VD, St Surin-Lord S, Davis EC, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol 2011;12:87-99.

5. Taylor S, Grimes P, Lim J, et al. Postinflammatory hyperpigmentation. J Cutan Med Surg 2009;13:183-91.



6. Bracaglia R, Tambasco D, Gentileschi S, et al. Patchy areolar hyperpigmentation 6 years after augmentation mastopexy: a case report. Aesthetic Plast Surg 2013;37:392-4.



7. Gabbi TV, Valente NY, Castro LG. Pigmented Paget’s disease of the nipple mimicking cutaneous melanoma: importance of the immunohistochemical profile to differentiate between these diseases. An Bras Dermatol 2006;81:457-60.

8. Patel AB. Postinflammatory hyperpigmentation: review of pathogenesis, prevention, and treatment. Pigment Int 2014;1:59.

9. Tomita Y, Maeda K, Tagami H. Melanocyte-stimulating properties of arachidonic acid metabolites: possible role in postinflammatory pigmentation. Pigment Cell Res 1992;5(5 Pt 2): 357-61.


10. Ruiz-Maldonado R, Orozco-Covarrubias ML. Postinflammatory hypopigmentation and hyperpigmentation. Semin Cutan Med Surg 1997;16:36-43.


11. Ortonne JP, Passeron T. Melanin pigmentary disorders: treatment update. Dermatol Clin 2005;23:209-26.


-
METRICS

-
- 0 Crossref
- 2,691 View
- 186 Download
- Related articles in AAPS
-
Malignant hyperthermia: a case report with a literature review2022 April;28(2)
Treatment of complications after augmentation mammoplasty.2000 March;6(1)
Submuscular periareolar approach to augmentation mammoplasty.2000 September;6(2)