 |
 |
- Search
| Arch Aesthetic Plast Surg > Volume 28(4); 2022 > Article |
|
Abstract
Background
This study aimed to evaluate the efficacy and safety of a dissolving microneedle (DMN)-encapsulated niacinamide skin patch to reduce facial hyperpigmentation.
Methods
A split-face study was conducted between April and June 2022 in 17 patients treated with a DMN-encapsulated niacinamide skin patch, which was applied only on the right side of the face, while the left face remained free of a pigmentation-improving agent. A topical moisturizer and physical sunscreen were applied on both sides of the face for 2 weeks. We compared both sides of the face 2 weeks after applying the skin patch using an automatic skin analysis device to investigate skin pigmentation. The melasma severity scores of both sides were evaluated before and 2 weeks after application.
Results
A significant difference in the epidermal pigmentation score between pre-treatment and 2 weeks after treatment was noted on the right side (P<0.05), but not on the left side of the face (P>0.05). A significant difference in the melanin score between pre-treatment and 2 weeks after treatment was noted on the right side (P<0.05), but not on the left side (P>0.05) of the face. There was no significant difference in the melasma severity score on either side of the face between pre-treatment and 2 weeks after treatment (P>0.05).
Facial hyperpigmentation plays an important role in facial appearance as people age. It is characterized by asymmetrical, irregular light brown to dark macules and patches on the malar area of the face [1]. Facial hyperpigmentation is caused by abnormal activation of melanocytes due to ultraviolet (UV) light exposure, hormonal changes, and genetic predisposition [1-3].
Vitamin C can lead to decreased melanin synthesis via the inhibition of tyrosinase [4]. Niacinamide is mainly used as a vitamin B3 supplement and has numerous cosmetic uses [5-7]. Niacinamide has a protective effect against UV-light-induced DNA damage in melanocytes by accelerating various signaling pathways [8]. Moreover, niacinamide leads to skin whitening by reducing the transfer of melanosomes from melanocytes to keratinocytes and by decreasing melanin synthesis [5,9].
Transdermal therapy, including creams, gels, and ointments, is an alternative modality to treat local diseases; however, it has limitations because the stratum corneum prevents the entrance of foreign compounds into the skin [10,11]. To overcome this skin barrier, microneedles have been designed to penetrate the skin barrier and deliver encapsulated active compounds. Dissolving microneedles (DMN) have recently been developed to enhance the efficacy of delivery during biodegradation without residual biohazardous materials [12,13].
To reduce facial hyperpigmentation, a DMN-encapsulated niacinamide skin patch was developed. The primary aim of this study was to evaluate the efficacy and safety of using a DMN-encapsulated niacinamide skin patch to reduce facial hyperpigmentation.
We conducted a split-face study to evaluate the efficacy of the DMN-encapsulated niacinamide skin patch. Of the patients who applied a DMN-encapsulated niacinamide skin patch to reduce facial hyperpigmentation between April and June 2022, 17 patients (Fitzpatrick skin type III-IV), who understood and agreed with the rationale and methodology of this study were enrolled. We excluded patients with a history of keloid scars, recent use of oral retinoids, pregnancy, immunosuppressive drug use, active systemic or local infections, and a history of psychiatric illness.
The study conformed to the principles of the Declaration of Helsinki, and written consent was obtained from each patient for both surgery and publication of photographs of the results. This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (IRB No. 2022-07-006).
Each side of the face was subjected to a different method of facial hyperpigmentation care to evaluate the efficacy of the DMN-encapsulated niacinamide skin patch (Keep Me Patch; SH Bio, Seoul, Korea) (Fig. 1), which was applied to the right side of the face for 3 hours. The patients were instructed to apply the DMN-encapsulated niacinamide skin patch to the right side of the face three times per week and a topical moisturizer and physical sunscreen to both sides of the face for 2 weeks. All patients were advised to avoid direct sunlight and use broad-spectrum sunscreen until the end of the study.
We evaluated the improvement in skin pigmentation on the face using an automatic skin analysis device (Mark-Vu; PSI Plus Co., Suwon, Korea) with UV light before and 2 weeks after facial hyperpigmentation care. The epidermal pigmentation score (EPS) and melanin score (MS) were analyzed using an automatic skin analysis device (Mark-Vu). The melasma severity score (MSS) has four grades of severity as follows: score 0, equivalent to surrounding normal skin or with minimal residual pigmentation; score 1, slightly darker; score 2, moderately darker; and score 3, markedly darker than the surrounding normal skin [14]. The MSS was evaluated by two blinded plastic surgeons (SMN and ESP).
Statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). The Wilcoxon signed-rank test was used to compare the improvement in skin pigmentation and MSS before and 2 weeks after facial hyperpigmentation care. Statistical significance was set at P<0.05.
Of the 17 patients with environment-induced skin pigmentation who were treated with a DMN-encapsulated niacinamide skin patch, 16 were female and one was male. The mean age of the patients was 35.5 years (range, 27–51 years) and the mean follow-up period was 4 weeks (range, 4–6 weeks) (Table 1). No complications, such as hyperpigmentation or skin allergy, were observed during the follow-up period.
Using a split-face study, we analyzed the efficacy of a DMN-encapsulated niacinamide skin patch in improving skin pigmentation (Fig. 2). The EPS and MS were analyzed using an automatic skin analysis device (Mark-Vu). On the right side of the face, which received a DMN-encapsulated niacinamide application skin patch, the EPS was 15.0 (interquartile range [IQR], 10.5–19.0) and 17.0 (IQR, 11.5–19.0) pre-treatment and 2 weeks post-treatment, respectively. The MS was 19.0 (IQR, 15.0–22.0) and 17.0 (IQR, 14.5–19.5) pre-treatment and 2 weeks post-treatment, respectively. On the left side of the face, which received only a topical moisturizer and physical sunscreen application, the EPS was 15.0 (IQR, 9.5–19.5) and 16.0 (IQR, 12.0–18.0) pre-treatment and 2 weeks post-treatment, respectively. The MS was 18.0 (IQR, 15.0–20.5) and 19.0 (IQR, 15.5–21.5) pre-treatment and 2 weeks post-treatment, respectively.
The EPS on the right side of the face was significantly different between pre- and post-treatment (P<0.05) but not on the left side of the face (P>0.05) (Fig. 3). The MS showed a significant difference between pre-treatment and post-treatment on the right side of the face (P<0.05), but not on the left side (P>0.05) (Fig. 4).
In addition, we evaluated the improvement in the MSS between both sides of the face. On the right side, the MSS was 1.0 (IQR, 1.0–2.0) and 1.0 (IQR, 1.0–1.5) pre-treatment and 2 weeks post-treatment, respectively, while on the left side of the face, the MSS was 1.0 (IQR, 1.0–1.5) and 1.0 (IQR, 1.0–2.0) pre-treatment and 2 weeks post-treatment, respectively. There were no significant differences on either side of the face between pre- and post-treatment (P>0.05) (Fig. 5).
Niacinamide is the biologically active form of niacin (vitamin B3), and it can be used as a cosmetic moisturizer. Niacinamide is a precursor of enzyme cofactor groups, such as nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate [15]. It may have various effects on the skin as an anti-inflammatory agent in acne, an antioxidant, and a compound that prevents photoimmunosuppression and photocarcinogenesis [16-18]. Niacinamide is stable in heat and light and is well tolerated in the skin, whereas other forms of the vitamin B3 family induce uncomfortable skin flushing reactions [15,19]. Recent research has reported that topical niacinamide inhibits the transfer of melanosomes from melanocytes to keratinocytes [5]. We treated patients with hyperpigmentation by applying niacinamide three times per week because the effect of niacinamide is reversible after 3 days [9]. We designed this study to evaluate the effects of niacinamide by referring to the results of the previous study.
Numerous transdermal drug delivery systems have recently been developed for effective local and systemic therapy [11]. In the field of skin aesthetics, solid microneedles have been developed to penetrate the skin and increase effective drug delivery via skin micro-channels. However, solid microneedles have safety concerns, such as reuse problems and infections [11]. To overcome these limitations of solid microneedles, DMNs have been developed. A DMN patch is an effective transdermal delivery system that contains encapsulated therapeutic drugs within their matrix and releases them into the skin during the biodegradation of DMNs [20]. The DMN used in our study consisted of trehalose and sodium hyaluronate, and did not contain sharp hazardous needle waste. In addition, the skin patch drug delivery system is a more patient-friendly treatment than that of other skin pigmentation treatment systems, such as lasers and hydroquinone application [11].
There was a significant difference in the EPS on the right side of the face, but not on the left side of the face. In particular, the EPS on the right side of the face deteriorated compared to its condition before treatment. A likely explanation is that post-inflammatory hyperpigmentation (PIH) occurred when the small wound, which occurred when the DMN penetrated the skin, healed. We speculate that this involved a mechanism similar to that of PIH after fractional CO2 laser treatment. Vitamin C is usually used to treat PIH; therefore, we assumed that the EPS could be improved if our study had a long-term follow-up period.
There was a significant improvement in the MS on the right side of the face, but not on the left side of the face. Based on these results, we conclude that the DMN-encapsulated niacinamide skin patch has the potential to improve skin pigmentation by inhibiting the transfer of melanosomes from melanocytes to keratinocytes [5]. There was no significant difference in the MSS between pre- and post-treatment on either side of the face. The MSS was evaluated by two blinded plastic surgeons who had difficulty identifying the change between pre- and post-treatment, and it is possible that they could not identify the improvement in skin pigmentation due to the short-term follow-up periods.
In this study, we evaluated the efficacy of a DMN-encapsulated niacinamide skin patch using a split-face study. The EPS of the right side of the face significantly worsened between pre-treatment and 2 weeks after treatment, and PIH seemed to result from DMN. The right side of the face, to which a DMN-encapsulated niacinamide skin patch was applied, exhibited a greater improvement in the MS than that of the left side, to which only topical moisturizer and physical sunscreen were administered. The differences in the MS improvement on the right side of the face were statistically significant, and we interpreted this finding as indicating that niacinamide inhibits the transfer of melanosomes from melanocytes to keratinocytes. We investigated the change in MSS scores on both sides of the face before and 2 weeks after treatment. However, there were no significant differences owing to the relatively short follow-up period. Based on our study, we believe that DMN-encapsulated niacinamide skin patches increase the early therapeutic effect of skin pigmentation through the inhibition of melanosome transfer, although PIH can be provoked by DMN at an early stage of treatment.
However, our study had some limitations. First, our study had a relatively short-term follow-up period; therefore, we did not evaluate full-term changes in skin pigmentation. Second, a small number of patients were enrolled in our study, which only included Asian populations with Fitzpatrick skin types III-IV. Therefore, we intend to conduct further studies with larger sample size, randomized design, relatively long-term follow-up period, and a broader population.
Despite these limitations, the application of a DMN-encapsulated niacinamide skin patch can provide a patient-friendly approach and an enhanced improvement in skin pigmentation.
CONFLICTS OF INTEREST
Seung Min Nam, Han Gyu Cha, and Eun Soo Park are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (IRB No. 2022-07-006) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and use of their images.
Fig. 1.
Photographic findings of the dissolving microneedle-encapsulated niacinamide skin patch (Keep Me Patch, SH Bio) (A) and dissolving microneedles (B). Magnified findings of a dissolving microneedle (C).
Fig. 2.
Clinical photographs of a 32-year-old female patient with skin pigmentation. Ultraviolet (UV) photographs of the left side of the face on which topical moisturizer and physical sunscreen were applied, pre-treatment (A) and 2 weeks after treatment (B). UV photographs of the right side of the face, to which a dissolving microneedle-encapsulated skin patch was applied, pre-treatment (C) and 2 weeks after treatment (D).

Fig. 3.
On the right side of the face (administered a dissolving microneedle-encapsulated niacinamide skin patch), the epidermal pigmentation score was 15.0 (interquartile range [IQR], 10.5–19.0) pre-treatment and 17.0 (IQR, 11.5–19.0) at 2 weeks after treatment. The difference between pre-treatment and 2 weeks after treatment was statistically significant (P<0.05). On the left side of the face (administered topical moisturizer and physical sunscreen), the epidermal pigmentation score was 15.0 (IQR, 9.5–19.5) pre-treatment and 16.0 (IQR, 12.0–18.0) 2 weeks after treatment. The difference was not statistically significant (P>0.05).

Fig. 4.
On the right side of the face (administered a dissolving microneedle-encapsulated niacinamide skin patch), the melanin score was 19.0 (interquartile range [IQR], 15.0–22.0) pre-treatment and 17.0 (IQR, 14.5–19.5) at 2 weeks after treatment. The difference between pre-treatment and 2 weeks after treatment was statistically significant (P<0.05). On the left side of the face (administered topical moisturizer and physical sunscreen), the melanin score was 18.0 (IQR, 15.0–20.5) pre-treatment and 19.0 (IQR, 15.5–21.5) at 2 weeks after treatment. The difference was not statistically significant (P>0.05).
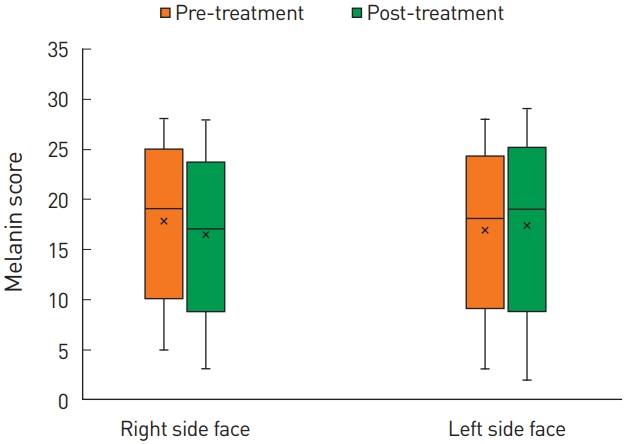
Fig. 5.
The melasma severity score between the right and left sides of the face. On the right side, the melasma severity score was 1.0 (interquartile range [IQR], 1.0–2.0) pre-treatment and 1.0 (IQR, 1.0–1.5) at 2 weeks after treatment. On the left side, the melasma severity score was 1.0 (IQR, 1.0–1.5) pre-treatment and 1.0 (IQR, 1.0–2.0) at 2 weeks after treatment. The difference was not significant on both sides of the face (P>0.05).

REFERENCES
1. Choi YJ, Nam JH, Kim JY, et al. Efficacy and safety of a novel picosecond laser using combination of 1 064 and 595 nm on patients with melasma: a prospective, randomized, multicenter, split-face, 2% hydroquinone cream-controlled clinical trial. Lasers Surg Med 2017;49:899-907.



3. Kang MS, Kim JH, Nam SM, et al. A split-face study evaluating the efficacy of a topical antioxidant cream containing tocotrienol after 1064-nm picosecond Nd:YAG laser treatment for environment-induced skin pigmentation. Arch Aesthetic Plast Surg 2021;27:100-5.


5. Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol 2002;147:20-31.


6. Ratcliffe DR, Iqbal J, Hussain MM, et al. Fibrillar collagen type I stimulation of apolipoprotein B secretion in Caco-2 cells is mediated by beta1 integrin. Biochim Biophys Acta 2009;1791:1144-54.


7. Virador VM, Kobayashi N, Matsunaga J, et al. A standardized protocol for assessing regulators of pigmentation. Anal Biochem 1999;270:207-19.


8. Chhabra G, Garvey DR, Singh CK, et al. Effects and mechanism of nicotinamide against UVA- and/or UVB-mediated DNA damages in normal melanocytes. Photochem Photobiol 2019;95:331-7.




9. Greatens A, Hakozaki T, Koshoffer A, et al. Effective inhibition of melanosome transfer to keratinocytes by lectins and niacinamide is reversible. Exp Dermatol 2005;14:498-508.


10. Bai JP, Amidon GL. Structural specificity of mucosal-cell transport and metabolism of peptide drugs: implication for oral peptide drug delivery. Pharm Res 1992;9:969-78.

11. Kang G, Kim S, Yang H, et al. Combinatorial application of dissolving microneedle patch and cream for improvement of skin wrinkles, dermal density, elasticity, and hydration. J Cosmet Dermatol 2019;18:1083-91.



12. Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008;29:2113-24.



13. Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res 2006;23:1008-19.



14. Kim J, Kim J, Lee YI, et al. Effect of a topical antioxidant serum containing vitamin C, vitamin E, and ferulic acid after Q-switched 1064-nm Nd:YAG laser for treatment of environment-induced skin pigmentation. J Cosmet Dermatol 2020;19:2576-82.



15. Lee DH, Oh IY, Koo KT, et al. Reduction in facial hyperpigmentation after treatment with a combination of topical niacinamide and tranexamic acid: a randomized, double-blind, vehicle-controlled trial. Skin Res Technol 2014;20:208-12.


16. Bowes J, Piper J, Thiemermann C. Inhibitors of the activity of poly (ADP-ribose) synthetase reduce the cell death caused by hydrogen peroxide in human cardiac myoblasts. Br J Pharmacol 1998;124:1760-6.




17. Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer 1997;29:157-62.


18. Tanno O, Ota Y, Kitamura N, et al. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br J Dermatol 2000;143:524-31.









