|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 29(1); 2023 > Article |
|
Abstract
Background
Enkephalin, an endogenous neuropeptide, binds to the delta (δ) opioid receptor and exerts an antinociceptive effect. Recent studies have suggested that neuropeptides might effectuate cutaneous wound healing. Therefore, we investigated the effects of an enkephalin derivative on wound healing and scar formation in vivo.
Methods
Enkephalin derivatives (leucine-enkephalin) were synthesized using the alanine scan method, and the most promising derivative (E10) was selected for further testing. In 15 C57BL/6N mice, two full-thickness skin defects (10 mm in diameter) were made on both sides of the back (left side, enkephalin group; right side, control group). The enkephalin group was administered 100 μL of E10 (AGGFL, 200 μg/mL), and the control group received phosphate-buffered saline. The wound size was digitally analyzed on days 2, 4, 7, and 10. After 21 days, the scar tissues were histologically evaluated for the scar depression index (SDI), and the epidermal growth factor (EGF) concentration was assessed using an enzyme-linked immunosorbent assay.
Results
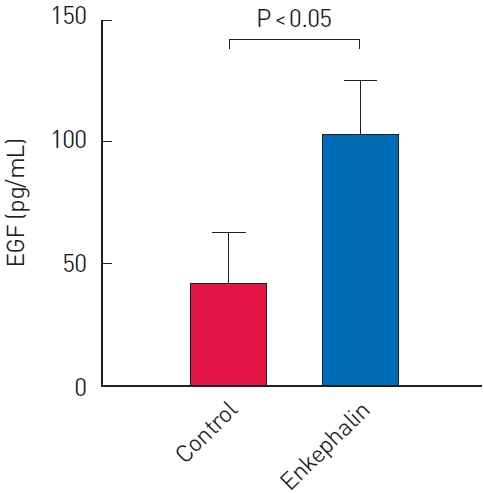
The skin defect percentages were 98.4%±17.9% (day 2), 83.2%±24.0% (day 4), 39.7%±17.4% (day 7), and 16.2%±10.0% (day 10) in the control group and 86.1%±15.0% (day 2), 61.4%±11.6% (day 4), 26.6%±8.8% (day 7), and 16.4%±8.8% (day 10) in the enkephalin group. The SDI values were significantly lower in the enkephalin group (0.06±0.19) than in the control group (0.22±0.13, P<0.001). The EGF level was significantly higher in the enkephalin group (102.2±22.6 pg/mL) than in the control group (42.1±20.5 pg/mL, P<0.001).
Wound healing, which refers to a range of mechanisms that restore the integrity and function of the skin, is a complex pathophysiological process involving the interplay of several cellular and biochemical processes [1]. This highly complex phenomenon includes the complex relationship between inflammation, re-epithelialization, angiogenesis, granulation tissue formation, and collagen deposition. Each process is regulated by a complex signaling network that is controlled by many growth factors, cytokines, and chemokines. Any impairment in the normal reparative process may lead to either delayed healing or inadequate fibrosis. Skin ulcers, including diabetic foot, venous, and pressure ulcers, are among the most frequent and specific types of chronic non-healing wounds. One of the major causes of delayed healing is the persistence of inflammation or an inadequate angiogenic response [2,3]. In contrast, depressed scars, hypertrophic scars, and keloids are characterized by abnormal production and degradation of collagen within the wound site during the healing process [4,5].
The development of medicine has increased life expectancy and, consequently, the prevalence of chronic diseases, such as hypertension, diabetes mellitus, and chronic wounds [6]. Various studies have been conducted on the treatment of chronic wounds to improve the quality of life. In clinical practice, dressing materials containing not only antibiotics but also growth factors and cytokines, and novel devices, such as negative-pressure wound therapy devices, have been developed [7]. In particular, various ointments and spray formulations using epidermal growth factor (EGF) have been developed, commercialized, and used to treat many chronic wounds and skin defects in clinical trials. It is thought that fast wound healing can help patients promptly return to daily life and benefit the economy. Hence, research on wound healing has significant value in light of the trend to emphasize quality of life, and efforts to enhance wound healing and develop novel dressing materials have continued to gain prominence. Increasing evidence has recently emerged that cutaneous innervation is an important modulator of the normal wound healing process due to the close interactions between the skin and peripheral nervous system [8].
Skin and nerve cells are both derived from the ectoderm during the development of the germ layers. Studies have suggested that neuropeptide receptors are expressed in the cellular membrane of the skin, and almost all neuropeptides are found in the skin [9]. According to Bigliardi-Qi et al. [10,11], deletion of the δ-opioid receptor delayed wound healing, while its activation promoted cutaneous wound healing in a mouse model. Enkephalin is an opioid peptide present in the forms of leucine-enkephalin (L-ENK) and methionine-enkephalin in the body. It acts as a ligand that binds to the μ- and δ-opioid receptors. The basic functions of enkephalin and δ-opioid receptors, including antinociceptive actions, are well known [12,13].
A clinical study recently reported that different neuropeptide receptors are found in various types of scar tissue [9]. Based on the evidence for wound healing and cicatrization discussed above, we hypothesized that an enkephalin derivative would promote wound healing and reduce scar formation. Enkephalin derivatives were derived from L-ENK using the alanine scan method. Our preliminary studies examined the effects of enkephalin on wound recovery in vitro and identified a promising enkephalin derivative [14,15]. Based on this, we created a full-thickness skin defect in a mouse model, investigated how quickly the wound recovered with L-ENK administration, and further investigated its effects on scar formation.
L-ENK (YGGFL) was synthesized and provided by BIO-FD&C, Co., Ltd. Based on L-ENK, AGGFL (E10) was synthesized using the conventional solid-phase peptide synthesis method with 9-fluorenylmethoxycarbonyl (Fmoc) as a protector of amino acids. Fmoc-Leu-Wang resin was used (Wang resin attached to leucine, which is an amino group protected by Fmoc). Extension of the amino acid residue was achieved by using N-hydroxybenzotriazole (HOBt) and N,N´-diisopropylcarbodiimide (DIC) as activators. The amino acids, HOBt, and DIC, used in the step-by-step binding process, were combined with five equivalents of resin and reacted at room temperature for 2 hours. After the reaction was complete, it was washed six times with dimethylformamide, six times with dichloromethane, and then dried. Furthermore, AGGFL-resin, a dried pentapeptide, was reacted with a combination of trifluoroacetic acid: triisopropylsilane: H2O (90:5:5 [v/v]) for 2 hours at room temperature to separate the peptide from the resin. Further, the peptide was precipitated with cold ether to obtain the crude peptide, using a centrifuge. Finally, the obtained AGGFL (E10) derivative was purified by reverse-phase high-performance liquid chromatography (column: Gemini, C18; 5 μ; 110 Å, 250×21.2 mm) using 0.1% trifluoroacetic acid and acetonitrile as the solvent. Other enkephalin derivatives were synthesized in the same manner as described above.
Fifteen C57BL/6N mice (Orient Bio Inc.), which were 6 weeks old and weighed 18 to 24 g, were used. All animal procedures were approved by the Institutional Animal Care and Use Committee. Mice were anesthetized with an intraperitoneal injection of 0.006 mL/10 g Zoletil and 0.004 mL/10 g Rompun. After hair removal, two full-thickness skin defects with a diameter of 10 mm were made on both sides of the back of the mouse. In each mouse, the right-side defect was treated with an enkephalin derivative (enkephalin group), and the left-side defect was treated with phosphate-buffered saline (PBS; control group). All skin defects were covered with a semipermeable film (Tegaderm; 3M Health Care), and prepared enkephalin was administered between the skin defect and semipermeable film using a syringe and a 26G needle. In the enkephalin group, 100 μL of E10 (AGGFL; 200 μg/mL) was injected into the skin defect site, and 100 μL of sterile PBS was injected in the control group. To evaluate changes in wound size, digital photographs were taken on days 2, 4, 7, and 10, starting from the first experimental day. The camera lens was positioned vertically on the wounds. The camera was fixed at a certain distance using a stand to minimize errors in the position of the camera. Digital images of the wound were analyzed, and the number of pixels in each wound was determined using ImageJ software (National Institutes of Health). The proportion of wounds on days 2, 4, 7, and 10 were calculated and compared to the initial defect size (Fig. 1).
The mouse excisional model described above was used to evaluate the effect of the enkephalin derivative on cicatrization. After 21 days, full-thickness scar tissue was harvested from the surrounding normal tissue. The tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Tissue sections were observed under a light microscope and evaluated using CaseViewer (3DHISTECH Ltd.). The width and thickness of the depressed scar were measured and expressed using the scar depression index (SDI) (Fig. 2). The SDI was defined as the difference in area between the unwounded dermis and wounded dermis divided by the unwounded dermis. The SDI value is less than 1 by definition, and a value closer to 0 indicates a lower degree of dermal depression.
Wounded skin fragments of the mice were homogenized in RIPA buffer (10 mM Tris-Cl, pH 8.0; 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, and 1 mM PMSF) containing a mixture of protease inhibitors for 30 minutes on ice. Homogenates were centrifuged at 20,000×g for 15 minutes at 4 °C, and supernatants were collected and measured using a Pierce protein bicinchoninic acid assay (Thermo Fisher). EGF production was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (PeproTech). Samples and standards (100 µL in each well) were added to washed plates in duplicate. The plates were then covered and incubated for 2 hours at room temperature. The plates were washed before the addition of the antibody detection solution and incubation for 2 hours at room temperature. The washed plates were incubated with streptavidin-horseradish peroxidase conjugate solution (Amersham) for 30 minutes. The plates were washed with PBS and incubated with 100 µL of tetramethylbenzidine solution for 20 minutes at room temperature. The staining reaction was stopped using a stop-solution, and absorbance was measured at 490 nm. EGF levels were expressed as picograms per milligram of total protein.
Statistical analyses were performed using the IBM SPSS version 20. Representative data were presented as mean±standard deviation. Statistical significance was set at P<0.05. The Kolmogorov-Smirnov test was used to verify data normality. The independent two-sample t-test and Mann-Whitney U test were used for parametric and non-parametric tests, respectively.
Based on L-ENK, enkephalin derivatives (AGGFL, YAGFL, YGAFL, YGGAL, and YGGFA) were synthesized using the alanine scan method by substituting the position of each amino acid with alanine (Table 1). Among these, E10 (AGGFL) and E13 (YGGAL) were the most effective in promoting wound healing in our preliminary studies [14,15]. Furthermore, a pilot test showed that after complete wound healing, the total collagen content of the E10 group was lower than that of the other groups. Therefore, only the E10 derivative was assessed in this study.
All 15 mice survived until day 10. In the control group, the percentage areas of the skin defects were 98.4%±17.9%, 83.2%±24.0%, 39.7%±17.4%, and 16.2%±10.0% on days 2, 4, 7, and 10, respectively. In the enkephalin group (E10 derivative), the percentage areas of the skin defects were 86.1%±15.0%, 61.4%±11.6%, 26.6%± 8.8%, 16.4%±8.8% on days 2, 4, 7, and 10, respectively. The wound healing rate of the enkephalin group was much higher than that of the control group, and the enkephalin group showed a statistically significant reduction in the wound area on days 2, 4, and 7 (P=0.023, P=0.006, and P =0.040, respectively) (Fig. 3A). Changes in the wound area over time were also confirmed in the gross photographs (Fig. 3B). The residual wound areas in the control group were statistically significantly larger on days 2, 4, and 7.
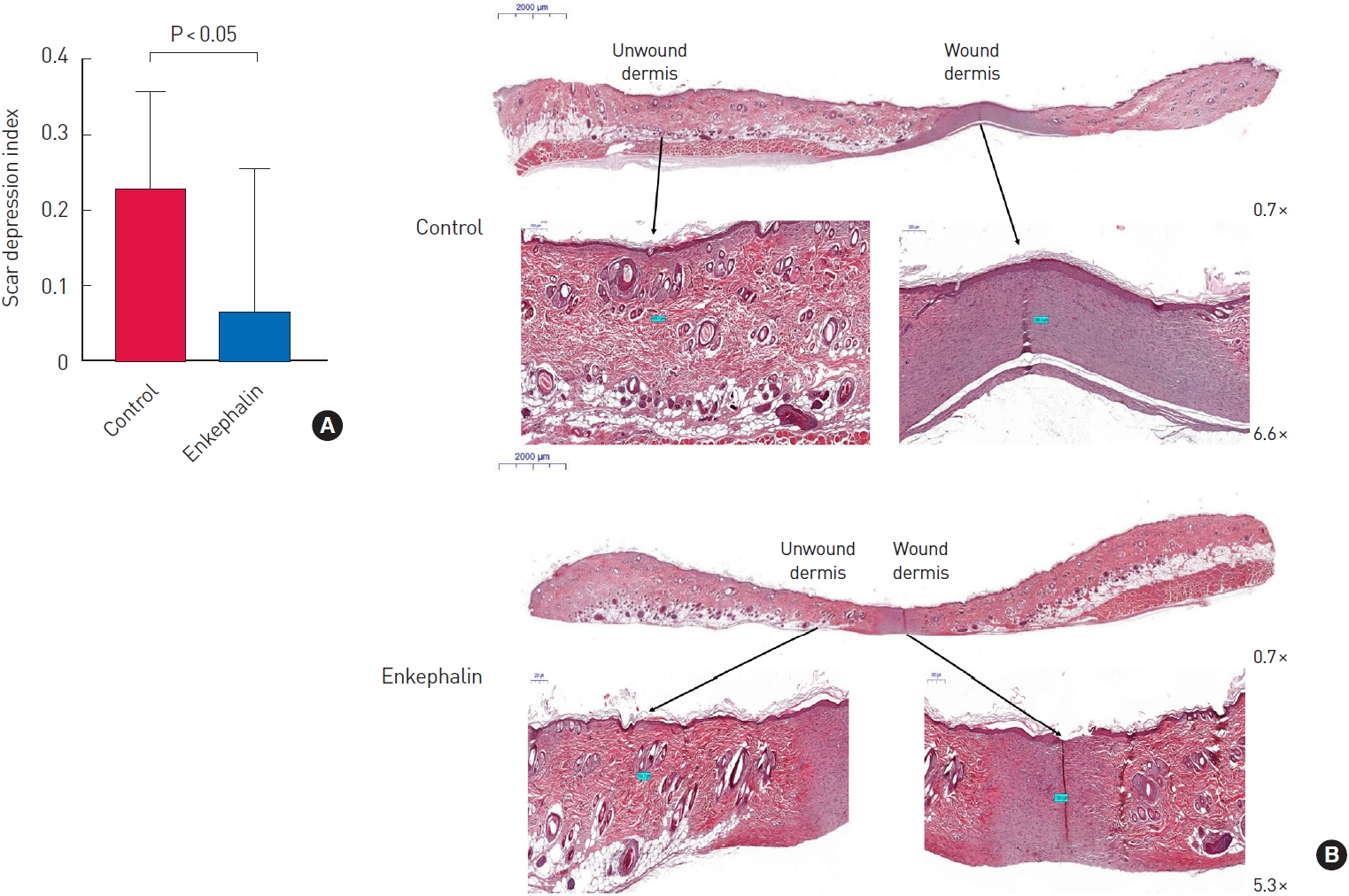
One of the 15 mice was lost before day 21, and the scar tissues of 14 mice were evaluated to prove the effectiveness of the enkephalin derivative on cicatrization. After the wound was completely healed on day 21, the SDI values were 0.22±0.13 in the control group and 0.06±0.19 in the enkephalin group. The SDI values of the enkephalin group were significantly lower than those of the control group (P<0.001) (Fig. 4A). Given the difference in SDI values, it could be interpreted that the enkephalin group had flatter skin surfaces in scar tissue. In the representative H&E-stained slides, the degree of depression of the wounded dermis between the two groups was markedly different (Fig. 4B).
The study was conducted using 14 samples from each group. To compare the concentrations of EGF in the enkephalin and control groups, an ELISA kit was used with excised scar tissues on day 21. The EGF levels of the control group and the enkephalin group were 42.1±20.5 pg/mL and 102.2±22.6 pg/mL, respectively, which showed a statistically significant difference (P<0.001) (Fig. 5).
In our preliminary studies, enkephalin derivatives were synthesized using the alanine scan method, and we investigated the dermal intracellular safety, enhancement of wound healing in cell migration assays, procollagen synthesis, and moisturizing performance of five enkephalin derivatives in vitro [15]. Of these five derivatives, we conducted an in vivo experiment with the E10 derivative, one of the most promising derivatives in our previous study, and found that the wounds healed quickly enough to be identified with the naked eye compared to the control group. To the best of our knowledge, this is one of the few preclinical in vivo studies in which opioids were involved in wound healing and showed a positive effect. In addition, our findings suggest that the atrophic scars were reduced in terms of SDI values, which might be partly associated with increased EGF levels.
Although various studies have been conducted on the effects of many neurotransmitters, such as enkephalin derivatives, on wound recovery, the precise biomolecular mechanism is unclear. However, the skin is abundant in various neurotransmitters and can affect wound recovery through interference with the complex signaling networks of different cells [8]. Additionally, in vitro studies have indicated that the mu (µ) opioid receptor/delta (δ) opioid receptor agonist, L-ENK, and its analogs can accelerate healing by fibroblast interaction, capillary growth, fibroblast proliferation, granulation tissue maturation, and epithelization [14,16,17]. Similarly, the increase in EGF concentration in the enkephalin group tissues in this study suggests that enkephalin derivatives stimulated wound healing directly by stimulating fibroblasts and increasing the synthesis of collagen matrixes [18]. These findings indicate the potential of these derivatives to replace commercially available EGF synthetic formulations, such as EGF ointments and sprays. If clinically applicable, neuropeptides as upstream modulators may be more effective in aiding homeostasis than EGF alone [11].
In this study, we showed that an enkephalin derivative improved wound healing in vivo, suggesting the possibility of its clinical application. Similarly, if enkephalin derivatives can promote wound healing in clinical practice, secondary infections could be prevented, and mortality or complications could be reduced in patients with many underlying diseases. As an increased emphasis is being placed on quality of life, rapid wound recovery would enable patients to return to their daily routine as soon as possible. In addition, highly efficient wound therapy can reduce the time until recovery and associated costs from an economic perspective [6]. Enkephalin derivatives can have antinociceptive actions in addition to wound healing, alleviating the pain that most patients with severe wounds experience [12,19,20]. Reducing pain can substantially improve patients’ quality of life. This is expected to be a major advantage compared to the synthetic EGF agents that are presently used in clinical practice.
In this study, the enkephalin derivative showed a positive effect on the inhibition of depressed scar formation after complete wound recovery. This indicates that the enkephalin derivative, as a cutaneous modulator, plays a role in both wound healing and the formation of scars. Currently, most early-stage scar products used after the complete epithelialization of a wound are dominated by silicone ointments or patches [21]. Enkephalin derivatives have the potential to replace these expensive scar products; however, large-scale clinical studies are required to confirm these findings. A clinical study reported that certain neuropeptide-containing nerves were found in painful hypertrophic human scar tissue, but no enkephalin-immunoreactive nerves were found [9]. This suggests that the enkephalin derivative analyzed in this study is a neuropeptide that may have a low probability of causing pain in scar tissue.
In this study, the increased EGF concentration in the scar tissue may have also played a role in the inhibition of scar formation. One clinical study showed that EGF levels and cicatrization were inversely proportional, whereas another showed that EGF in surgical wound healing may improve cutaneous scar quality [22,23]. In another preclinical study, a murine model of full-thickness wound healing suggested that EGF can reduce cutaneous scars by suppressing inflammatory reactions, decreasing the expression of transforming growth factor-β1, and mediating collagen formation [24]. Another preclinical study analyzed a rat model during the repair of depressed scars and found that autologous skin fibroblasts enhanced collagen production and filled depressed scars, which were also stimulated by EGF [25]. Based on these results, we can assume that, in our study, EGF played a key role in scar remodeling, which was stimulated by enkephalin derivatives.
This study has the following limitations. First, investigating the effects of cicatrization may require longer follow-up periods, as a longer period may lead to the formation of a hypertrophic scar rather than a depressed scar. In the mouse model, scar models are very diverse, such as incisional scars, excisional scars, and scar repair models; however, there is no research on which is optimal as a human scar model, so research will be needed in various scar models to be widely applied to the human body. As there are few studies on the effects of enkephalin derivatives on cicatrization, research is needed on various growth factors and cytokines other than EGF. In future studies, it is necessary to study the long-term effects, causes, and mechanisms of these factors in different scar models by supplementing the above descriptions.
In this mouse model study, an enkephalin derivative showed a positive effect of inducing wound healing more quickly and inhibiting depressed scar formation, which was partly associated with an increase in EGF concentration in scar tissues.
Notes
Ethical approval
The study was approved by the Lee Gil Ya Cancer and Diabetes Institutional Animal Care and Use Committee (LCDI-2014-0012).
Fig. 1.
Schematic diagram. Two full-thickness skin defects with a 10-mm diameter were made on both sides of the backs of 15 mice. The control sites on the left side and the enkephalin group on the right side were injected with 100 μL of sterile phosphate-buffered saline and the E10 (AGGFL) enkephalin derivative, respectively. To identify the effect of enkephalin derivatives on wound healing, the size of the wound was measured on days 2, 4, 7, and 10 using a digital camera and ImageJ software. On day 21, scar tissues were used to evaluate the scar depression index and epidermal growth factor (EGF) levels were assessed with an enzyme-linked immunosorbent assay (ELISA).
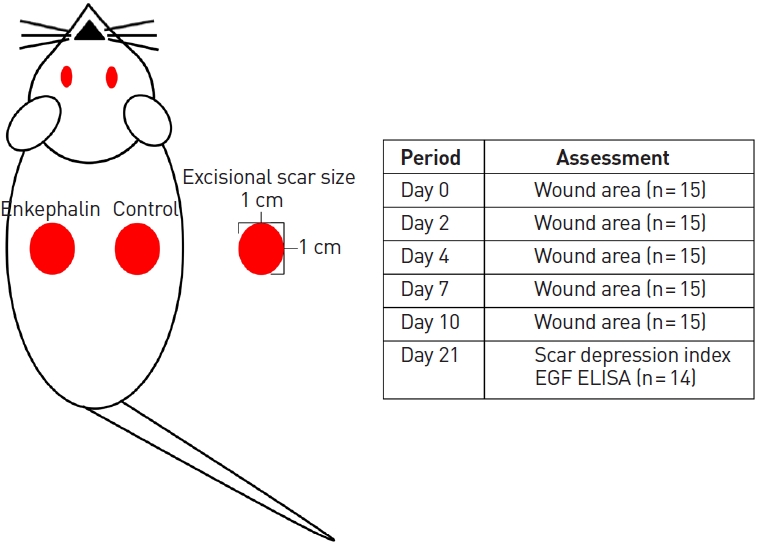
Fig. 2.
Scar depression index (SDI). In this study, the degree of pressure was expressed as the SDI to compare the degree of scar depression. The SDI was defined as the difference in area between the unwounded dermis and wounded dermis divided by the unwounded dermis. The SDI value is less than 1, and values closer to 0 indicate a lower degree of dermal depression.

Fig. 3.
Comparison of wound area between the control and the enkephalin groups (n=15 per group). (A) In the defect sites with the E10 derivative, the wounds healed at a very rapid rate from day 2, and the slope of the wound healing rate was relatively steep compared to the control group throughout the experimental period. On days 2, 4, and 7, the enkephalin group showed a smaller wound area than the control group and the difference was statistically significant (P<0.05). (B) It was also revealed that the enkephalin group (left) healed faster than the control group (right) during the 10-day experimental period, showing a noticeable difference on days 2, 4, and 7.
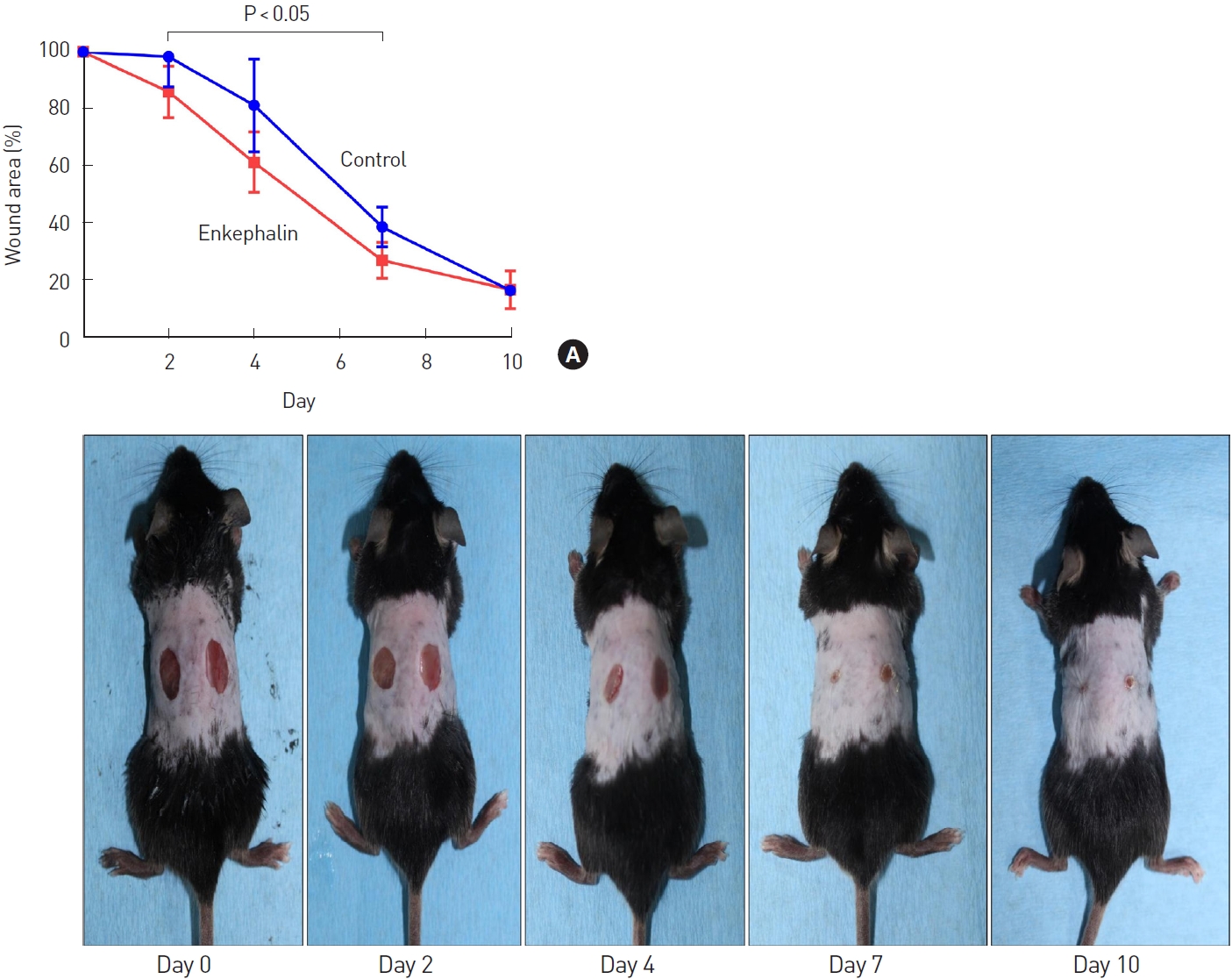
Fig. 4.
Histologic comparison of depressed scar between the control and the enkephalin group. (A) Comparison of the scar depression index (SDI) between the control and the enkephalin groups (n=14 per group). After complete wound healing at 21 days, the SDI values were 0.06±0.19 in the enkephalin group and 0.22±0.13 in the control group. The SDI values of the enkephalin group were significantly lower than those of the control group (P<0.001). (B) Comparison of representative histologic findings between the control and the enkephalin groups. As shown using hematoxylin and eosin staining, the degree of depression of the wounded dermis was severe in the control group, while the degree of depression of the wounded dermis was lower in the enkephalin group (magnification is recorded in the lower right corner of each photo).
REFERENCES
1. Rodrigues M, Kosaric N, Bonham CA, et al. Wound healing: a cellular perspective. Physiol Rev 2019;99:665-706.



4. Moon J, Yoon JY, Yang JH, et al. Atrophic acne scar: a process from altered metabolism of elastic fibres and collagen fibres based on transforming growth factor-β1 signalling. Br J Dermatol 2019;181:1226-37.



5. Limandjaja GC, Niessen FB, Scheper RJ, et al. Hypertrophic scars and keloids: overview of the evidence and practical guide for differentiating between these abnormal scars. Exp Dermatol 2021;30:146-61.



6. Phillips T, Stanton B, Provan A, et al. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31:49-53.


7. Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014;22:569-78.



8. Cheret J, Lebonvallet N, Carre JL, et al. Role of neuropeptides, neurotrophins, and neurohormones in skin wound healing. Wound Repair Regen 2013;21:772-88.


9. Crowe R, Parkhouse N, McGrouther D, et al. Neuropeptide-containing nerves in painful hypertrophic human scar tissue. Br J Dermatol 1994;130:444-52.


10. Bigliardi-Qi M, Gaveriaux-Ruff C, Zhou H, et al. Deletion of delta-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation 2006;74:174-85.


11. Bigliardi-Qi M, Bigliardi PL. The role of opioid receptors in migration and wound recovery in vitro in cultured human keratinocytes and fibroblasts. Methods Mol Biol 2015;1230:273-7.


12. Holden JE, Jeong Y, Forrest JM. The endogenous opioid system and clinical pain management. AACN Clin Issues 2005;16:291-301.


13. Henry MS, Gendron L, Tremblay ME, et al. Enkephalins: endogenous analgesics with an emerging role in stress resilience. Neural Plast 2017;2017:1546125.




14. Yang DJ, Lee KS, Ko CM, et al. Leucine-enkephalin promotes wound repair through the regulation of hemidesmosome dynamics and matrix metalloprotease. Peptides 2016;76:57-64.


15. Kim YW, Kim HS, Kim SY, et al. Development of scar improving materials using enkephalin derivatives. J Korea Acad Ind Coop Soc 2015;16:5336-42.

16. Vinogradov VA, Spevak SE, Iarygin KN, et al. Opioid activity of peptides and wound healing of the skin. Biull Eksp Biol Med 1987;104:89-91.

17. Kohl A, Werner A, Buntrock P, et al. The effect of the peptide dalargin on wound healing. Dermatol Monatsschr 1989;175:561-72.

18. Smith KD, Wells A, Lauffenburger DA. Multiple signaling pathways mediate compaction of collagen matrices by EGF-stimulated fibroblasts. Exp Cell Res 2006;312:1970-82.


19. Guedon JM, Zhang M, Glorioso JC, et al. Relief of pain induced by varicella-zoster virus in a rat model of post-herpetic neuralgia using a herpes simplex virus vector expressing enkephalin. Gene Ther 2014;21:694-702.




20. Sobczak M, Pilarczyk A, Jonakowski M, et al. Anti-inflammatory and antinociceptive action of the dimeric enkephalin peptide biphalin in the mouse model of colitis: new potential treatment of abdominal pain associated with inflammatory bowel diseases. Peptides 2014;60:102-6.


21. Kim JS, Hong JP, Choi JW, et al. The efficacy of a silicone sheet in postoperative scar management. Adv Skin Wound Care 2016;29:414-20.


22. Satici A, Guzey M, Dogan Z, et al. Relationship between Tear TNF-alpha, TGF-beta1, and EGF levels and severity of conjunctival cicatrization in patients with inactive trachoma. Ophthalmic Res 2003;35:301-5.



23. Shin JU, Kang SW, Jeong JJ, et al. Effect of recombinant human epidermal growth factor on cutaneous scar quality in thyroidectomy patients. J Dermatolog Treat 2015;26:159-64.