|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 29(1); 2023 > Article |
|
Abstract
Background
Implant-based breast reconstruction is a technique frequently used for breast reconstruction. Infection and inflammation are considered to be the most troublesome complications of implant-based breast reconstruction and can lead to capsular contracture or implant failure. To date, however, only a few methods have been proposed to prevent these complications. Therefore, the authors introduce a simple irrigation system using indwelling drain catheters to decrease postoperative inflammation.
Methods
Continuous saline irrigation was performed once per day for 3 days immediately after prosthesis-based breast reconstruction. Normal saline (500 mL) was inserted into the implant pocket through a superomedial-oriented drain catheter and drained through an inferolateral-oriented drain catheter using a suction device. Inflammatory indicators, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), and postoperative complications were compared between the non-irrigation and irrigation groups.
Results
This study included 37 patients divided into two groups (20 non-irrigation and 17 irrigation). An analysis of inflammatory indicators revealed that the peak CRP level in the irrigation group was significantly lower than that in the non-irrigation group, while no statistically significant differences were found for the other mediators (ESR and WBC). In the immediate postoperative period, continuous irrigation effectively washed out tissue debris and blood clots within the implant pocket, which helped maintain the function of the drain catheter and decrease pro-inflammatory mediators.
Implant-based breast reconstruction (IBR) is the most common method of breast reconstruction in the United States, consistently accounting for >70% of all breast reconstructions annually [1]. Of 109,256 breast reconstruction procedures, IBRs accounted for 79,019 [2]. Although IBR is undoubtedly popular among both surgeons and patients, it has disadvantages, including periprosthetic infections and capsular contracture, compared with autologous tissue transfer [3,4].
Periprosthetic infections represent the most devastating complication of IBR, with a reported rate of occurrence ranging from 1% to 35.4% of patients [5,6]. In addition, capsular contracture, which is caused by an exaggerated healing response of the fibrous capsule around an implant, results in negative aesthetic outcomes of breast augmentation and IBR procedures. Clinical studies by implant manufacturers over a period of 3 to 6 years reported a cumulative incidence of 6% to 18% for capsular contracture [7-9], which is clinically manifested by pain or discomfort of varying degrees caused by distortion and displacement of the implant, as reflected by the change in consistency and shape of the operated breast [10]. The etiology of capsular contracture remains unclear; however, recent reports have proposed two main hypotheses. First, the infectious process hypothesis describes a chronic subclinical infection located immediately adjacent to the implant sheath in a microscopic biofilm, which acts as a bacterial infection trigger for an inflammatory response. This process is provoked by contamination [11]. Despite the absence of definitive evidence of infection, excessive inflammation is considered to be one cause of capsular contracture. Second, hypertrophic scarring, a mechanism that involves stimulation of myofibroblasts present in the capsular tissue, determines the future formation of a contractile periprosthetic hypertrophic scar due to an inflammatory reaction toward foreign bodies [12,13]. Stimuli, such as hematoma or seroma, can trigger an inflammatory reaction.
Some procedures have been proposed for the treatment of periprosthetic infections and capsular contractures, including antibiotic breast pocket irrigation, the use of textured implants, and subpectoral implant placement. Currently, however, there is no definitive method to reduce these complications [14,15], although irrigation of the surgical site has been used for this purpose. Prompted by the first descriptions of the use of continuous wound irrigation to prevent wound infection by Carrel and Dehelly [16], Kasdan and Chipman [17] proposed a sophisticated irrigation system using a polyurethane catheter with cold lactated Ringer’s solution to continuously flush the surgical site during the immediate postoperative period following Dupuytren’s contracture release.
However, to our knowledge, no reports have described objective measurements of the anti-inflammatory effects of irrigation systems. In the present article, we present a simple irrigation system that uses an indwelling catheter to wash out the prosthetic pocket immediately after IBR. We analyzed serum levels of inflammationassociated markers including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and white blood cell (WBC) count.
A retrospective chart review was performed to evaluate the effectiveness of postoperative continuous irrigation via a catheter in patients who underwent immediate IBR between August 2020 and September 2021. Patients who underwent bilateral breast reconstruction, delayed reconstruction, neoadjuvant chemotherapy, or postmastectomy radiotherapy were excluded. Ultimately, 37 patients who underwent immediate IBR following total mastectomy were enrolled. All reconstructions were performed by a single surgeon (EH), whereas mastectomies were performed by two different breast surgeons.
Patients were divided into two groups: non-irrigation (n=20), patients who received standard postoperative care; and irrigation (n=17), patients who underwent closed irrigation through the drain catheter until postoperative day 3. All patients were evaluated for patient demographics, comorbidities, postoperative complications, and serum levels of inflammation-associated markers including CRP, ESR, and WBC. The personal data presented in this article were anonymized.
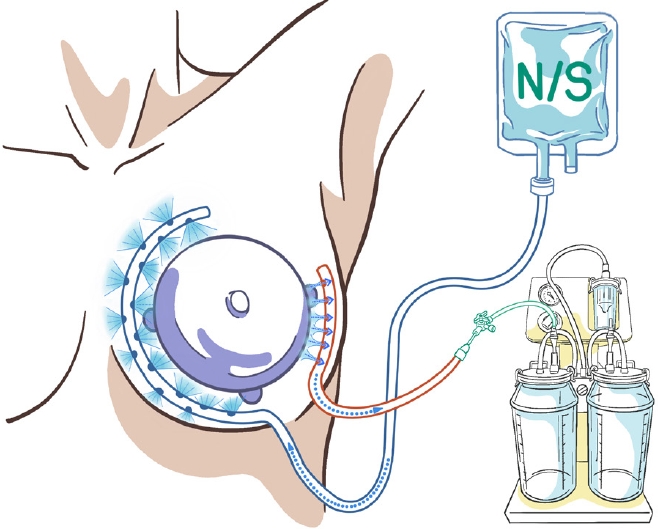
Thirty reconstruction cases, except for seven direct-to-implant reconstructions, involved two-stage IBR using a tissue expander (Mentor CPX 4 - SILTEX with Suture Tabs - Breast Tissue Expander; Johnson & Johnson). Two types of acellular dermal matrix (CG CryoDerm; CG Bio, and MegaDerm; L&C Bio) were used. Two Jackson-Pratt drains were placed in the inferolateral (drain A) and superomedial sides through the inframammary fold (drain B). Drain B was placed on the superomedial side of the implant pocket. The drain was removed once <30 mL/day of fluid was collected for 2 consecutive days. All patients received the same postoperative care except for irrigation.
The immediate postoperative continuous irrigation system included a 500 mL bag of 0.9% normal saline with sterile pressure tubing in an intravenous line extension kit. A three-way tap was used to connect a 500 mL bag of 0.9% normal saline to drain B, and a portable electric surgical suction pump (basic suction pump; Medela) was connected to drain A. The irrigation solution was inserted into drain B at a maximum drip rate and simultaneously suctioned out through drain A (Fig. 1). This system was used once daily for 3 days after surgery (Supplementary Video 1).
Perioperative surgical-site complications were also assessed. Inflammation-associated markers, including CRP, ESR, and WBC, were analyzed preoperatively and on postoperative days 1, 2, 3, and 7 to evaluate whether irrigation affected the levels of inflammation. Acute postoperative complications were defined as those occurring up to 90 days after reconstructive surgery and included surgical site infection, hematoma, mastectomy flap necrosis, implant failure, and wound dehiscence.
The normality of the distribution of the continuous variables was tested using the Shapiro-Wilk test, which showed non-normal distributions. The Mann-Whitney U test was used to generate exact P-values for comparing the distribution of independent samples. Continuous variables are expressed as median with interquartile range (Q1–Q3); categorical variables are expressed as frequencies and proportions. Differences with a P-value <0.05 were considered to be statistically significant. The statistical analyses were performed using SPSS version 22.0 (IBM Corp.) for Windows (Microsoft Corp.).
In total, 37 IBR procedures were analyzed and compared between the two groups. The patient and treatment characteristics, including potential risk factors for postoperative complications, are summarized in Table 1. There were no significant differences between the two groups. The mean follow-up was 299.4 days (range, 203–414 days) for the entire cohort, 321.6 days for the non-irrigation group (range, 204–414 days) and 272.9 days for the irrigation group (range, 203–363 days). On average, the drain indwelling time was 11.15 days in the non-irrigation group versus 10.64 days in the irrigation group.
Major surgical site infections, defined as those requiring additional surgical procedures, occurred in two cases in the non-irrigation group. The first was a patient who underwent immediate twostage IBR and developed fever and erythema at the surgical site on a postoperative day 20. Cultures remained negative for each fluid washout; however, clinical symptoms continued, ultimately requiring explantation and autologous breast reconstruction, with final cultures positive for Corynebacterium striatum. A patient who underwent immediate two-stage IBR required unplanned readmissions for fever and erythema on a postoperative day 27, with positive cultures for methicillin-resistant Staphylococcus epidermidis. This case required explantation and autologous breast reconstruction after infection clearance. No failed reconstructions were observed. However, none of these cases developed in the irrigation group. Seroma occurred in four cases in the non-irrigation group but developed in only one case in the irrigation group (20% vs. 6%). Hematoma occurred in one case in the non-irrigation group, but developed in no cases in the irrigation group (5% vs. 0%). Other minor postoperative complications related to delayed wound healing, such as dehiscence and partial flap necrosis, occurred at a similar frequency in both groups (Table 2). The mean duration of this irrigation procedure was 6 minutes and 37 seconds.
Laboratory investigation results, including the CRP, ESR, and WBC count as inflammation-associated markers, were also reviewed in both groups (Table 3). CRP peaked on a postoperative day 2 (3.98 mg/dL [interquartile range, 2.81–5.44 mg/dL] vs. 2.13 mg/dL [interquartile range, 1.53–3.01 mg/dL], P=0.004), and the mean peak CRP level in the irrigation group was significantly lower than that in the non-irrigation group. CRP levels normalized earlier in the irrigation group than in the non-irrigation group. There were no significant differences in the ESR and WBC count between both groups (Fig. 2). The serum levels of inflammatory mediators did not increase abnormally because the majority of the patients did not experience infections.
IBR is a relatively simple reconstruction technique; as such, it has become popular. However, it has some pitfalls, including capsular contracture and surgical site infection, which are major drawbacks of IBR. Therefore, several methods have been proposed to prevent perioperative infections in IBR procedures.
Currently, antibiotic prophylaxis in IBR includes the use of perioperative antibiotics and single irrigation in the implant pocket with a triple antibiotic cocktail [18,19]. However, the rate of postoperative infections still remains a significant concern after IBR [20]. In this study, we proposed a protocol for saline irrigation of the breast pocket to reduce blood clots, tissue debris, and inflammation-associated markers within the implant pocket. We found decreases in the incidence of surgical site infection, hematoma, and seroma in the irrigation group compared with the non-irrigation group.
Previous studies have already described the implementation of catheter-based continuous irrigation for multiple primary goals including the salvage of infected cartilage and the reduction of hematoma in Dupuytren’s contracture release. Furthermore, Tutela et al. [21] reported the effectiveness of a catheter-based continuous antibiotic irrigation system in reducing complications such as surgical site infection and premature explantation following immediate breast reconstruction using tissue expanders. Hunsicker et al. [22] also reported that continuous antibiotic irrigation of the peri-implant space with a triple antibiotic solution after IBR was significantly effective in reducing the incidence of postoperative infections. They demonstrated the effectiveness of continuous irrigation as an adjuvant therapeutic modality for reducing inflammation and other complications.
However, there are several differences between our procedure and those of previous reports. First, unlike previous studies for the treatment of postoperative complications, we used continuous irrigation for the prophylaxis of postoperative complications. Second, we only used a normal saline solution, whereas Tutela et al. [21] and Hunsicker et al. [22] used a triple antibiotic solution (cefazolinbased and vancomycin-based, respectively). As mentioned above, the current antibiotic prophylaxis regimen in IBR is a triple antibiotic cocktail. However, Drinane et al. [23] recently reported that there was no difference between a triple antibiotic solution and saline irrigation in the incidence or severity of capsular contracture. Most patients with clinical infections also had no bacterial growth in culture of seroma fluid, indicating sterile inflammation [24]. Given the accumulated evidence, we believe that saline irrigation also may be effective enough to mitigate the risk of postoperative hematoma, infection, and pain following the procedure. Third, we investigated the anti-inflammatory effects of continuous saline irrigation by a laboratory analysis of inflammatory mediators. In this study, inflammation-mediated marker analysis revealed a lower peak in CRP levels and a faster normalization pattern in the irrigation group than in the non-irrigation group. Although our cohort was small and inflammation-associated markers via blood samples could not instantly reflect the status of the implant pocket, we think that continuous saline irrigation may have contributed to systemic effects by affecting the physical and biological environment in the implant pocket. The underlying mechanism of continuous saline irrigation is considered to be a combination of factors.
Watt-Boolsen et al. [25] reported that seroma formation was a result of an inflammatory process in seroma fluid. In addition, Szecsi et al. [24] investigated the relationship between cytokine profiles in seroma fluid after mastectomy and the likelihood of seroma development, and found that seroma formation was most likely a proinflammatory process, as indicated by very high levels of interleukin (IL)-6 and IL-8. Although it has not been clearly established whether these findings have a causal relationship with postoperative complications, it is reasonable to hypothesize that suppressing inflammation may be a persuasive prophylactic measure. We speculate that irrigation provides not only a mechanical cleansing effect by washing out blood clots and tissue debris, but also a biological effect of diluting pro-inflammatory mediators (e.g., IL-6 and IL-8) within the implant pocket. Eventually, this may reduce inflammation and inflammation-related complications in IBRs.
In summary, we think that continuous saline irrigation is desirable for all patients who have undergone implantable device-based breast reconstruction including permanent implants or tissue expanders. However, if it is slightly laborious and time-consuming in actual clinical practice, it can be indicated for surgical cases in which excessive inflammation is expected or should be prevented in the breast pocket, including cases of suspected hematoma immediately after surgery, excessive tissue debris after autologous breast reconstruction, and direct-to-implant breast reconstruction.
The limitations of the present study include non-randomization, small sample size, and a heterogeneous sample population. Moreover, it did not analyze serous fluid levels of pro-inflammatory mediators such as IL-6 and IL-8. As such, further research investigating changes in the concentration of pro-inflammatory mediators after irrigation would be warranted.
In conclusion, catheter-based continuous irrigation effectively washed out physical debris and pro-inflammatory cytokines from the implant pocket and helped to maintain the function of the drain catheter and decrease inflammation in the immediate postoperative period. We believe that this irrigation method is quick, simple, and helpful in decreasing inflammation within the breast pocket.
Notes
Ethical approval
The study was approved by the Institutional Review Board of the CHA University Bundang CHA Medical Center (IRB No. 2022-09-065) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and use of their images.
Supplemental material
Supplementary materials can be found via https://doi.org/10.14730/aaps.2022.00724
Fig. 2.
Laboratory analyses of inflammation-associated markers. (A) The average level of C-reactive protein (CRP; mg/dL). (B) The average level of erythrocyte sedimentation rate (ESR; mm/hr). (C) The average level of white blood cell count (WBC; 103/μL). POD, postoperative day. a)Significant difference (P<0.05).

Table 1.
Patient demographics and perioperative surgical details
Table 2.
Complication profiles in the non-irrigation and irrigation groups
Table 3.
Laboratory analysis of inflammation-associated markers in the non-irrigation and irrigation groups
|
CRP (mg/dL) |
ESR (mm/hr) |
WBC (103/μL) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-irrigation | Irrigation | P-value | Non-irrigation | Irrigation | P-value | Non-irrigation | Irrigation | P-value | |
| Preoperative | 0.07 (0.06–0.10) | 0.09 (0.05–0.21) | 0.598 | 7.00 (4.75–12.00) | 7.00 (5.00–11.00) | 0.964 | 5.23 (4.77–6.45) | 6.77 (6.23–7.52) | 0.028a) |
| POD1 | 2.02 (1.58–3.15) | 1.82 (1.22–2.19) | 0.209 | 5.00 (2.00–10.50) | 8.00 (2.00–15.00) | 0.391 | 8.63 (7.63–9.83) | 9.91 (8.40–11.93) | 0.149 |
| POD2 | 3.98 (2.81–5.44) | 2.13 (1.53–3.01) | 0.004a) | 19.50 (10.25–25.25) | 9.50 (6.75–15.00) | 0.048a) | 6.96 (5.51–8.48) | 6.46 (5.64–8.69) | 0.775 |
| POD3 | 2.53 (1.29–3.03) | 1.51 (0.97–2.37) | 0.117 | 15.00 (9.50–27.75) | 17.00 (13.00–27.00) | 0.517 | 5.83 (4.90–7.11) | 5.80 (4.88–7.37) | 0.752 |
| POD7 | 0.29 (0.22–0.66) | 0.31 (0.19–0.60) | 0.752 | 11.00 (7.00–17.00) | 12.50 (9.75–20.00) | 0.424 | 5.50 (4.87–6.31) | 5.74 (3.79–6.95) | 0.988 |
REFERENCES
1. Xue AS, Volk AS, DeGregorio VL, et al. Follow-up study: one-step salvage of infected prosthetic breast reconstructions using antibiotic-impregnated polymethylmethacrylate plates and concurrent tissue expander exchange. Plast Reconstr Surg 2020;145:240e-250e.


2. American Society of Plastic Surgeons (ASPS). 2018 Plastic Surgery Statistics Report [Internet]. ASPS; c2017 [cited 2022 Oct 21]. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf
3. Eo PS, Lee JS, Lee JW, et al. Usefulness of meshed SurgiMend in direct-to-implant breast reconstruction. Arch Aesthetic Plast Surg 2021;27:69-75.


4. Rhie JW, Kim TS, Yoon HY, et al. Breast reconstruction using implant: long term follow-up complications & patient’s satisfaction. Arch Aesthetic Plast Surg 2014;20:36-43.

5. Peled AW, Stover AC, Foster RD, et al. Long-term reconstructive outcomes after expander-implant breast reconstruction with serious infectious or wound-healing complications. Ann Plast Surg 2012;68:369-73.


6. Bennett SP, Fitoussi AD, Berry MG, et al. Management of exposed, infected implant-based breast reconstruction and strategies for salvage. J Plast Reconstr Aesthet Surg 2011;64:1270-7.


7. Stevens WG, Harrington J, Alizadeh K, et al. Five-year follow-up data from the U. S. clinical trial for Sientra’s U.S. Food and Drug Administration-approved Silimed® brand round and shaped implants with high-strength silicone gel. Plast Reconstr Surg 2012;130:973-81.
8. Cunningham B, McCue J. Safety and effectiveness of Mentor’s MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4.



9. Spear SL, Murphy DK, Slicton A, et al. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg 2007;120(7 Suppl 1): 8S-16S.

10. Kim TH, Yoon SM, Wee SY, et al. A comparative discussion of incisional methods in total capsulectomy of the breast. Arch Aesthetic Plast Surg 2021;27:117-24.


11. Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg 2010;126:835-42.


12. Hunt J, Salomon J. Augmentation mammoplasty. Selected Read Plast Surg 2002;9:1-35.
13. Restifo RJ. A case report of capsular contracture immediately following COVID-19 vaccination. Aesthet Surg J Open Forum 2021;3:ojab021.




14. Zambacos GJ, Nguyen D, Morris RJ. Effect of povidone iodine on silicone gel breast implants in vitro: implications for clinical practice. Plast Reconstr Surg 2004;114:706-12.


15. Embrey M, Adams EE, Cunningham B, et al. A review of the literature on the etiology of capsular contracture and a pilot study to determine the outcome of capsular contracture interventions. Aesthetic Plast Surg 1999;23:197-206.



16. The treatment of infected wounds. By A. Carrel and G. Dehelly. Second English edition. 81 figures and 6 plates. 1918. London: Baillière, Tindall & Cox. 6s. net. Br J Surg 1918;6:475.


17. Kasdan ML, Chipman JR. Dupuytren’s contracture: wound irrigation to prevent hematoma. Orthop Rev 1987;16:525-8.

18. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195-283.



19. Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg 2006;117:30-6.


20. Phillips BT, Halvorson EG. Antibiotic prophylaxis following implant-based breast reconstruction: what is the evidence? Plast Reconstr Surg 2016;138:751-7.


21. Tutela JP, Duncan DP, Kelishadi SS, et al. Continuous postoperative antibiotic irrigation via catheter system following immediate breast reconstruction. Eplasty 2015;15:e49.


22. Hunsicker LM, Chavez-Abraham V, Berry C, et al. Efficacy of vancomycin-based continuous triple antibiotic irrigation in immediate, implant-based breast reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1624.



23. Drinane JJ, Kortes MJ, Bergman RS, et al. Evaluation of antibiotic irrigation versus saline irrigation in reducing the long-term incidence and severity of capsular contraction after primary augmentation mammoplasty. Ann Plast Surg 2016;77:32-6.