 |
 |
- Search
| Arch Aesthetic Plast Surg > Volume 24(1); 2018 > Article |
|
Abstract
Background
Gore-Tex implants started out as a product with the distinct advantage of producing a natural nose shape, but using Gore-Tex, it is difficult to predict the height of the nose after rhinoplasty because Gore-Tex contracts over time, making the nose shrink. However, Surgiform, a new form of expanded polytetrafluoroethylene (ePTFE) implant, enables prediction of the height of the nose after rhinoplasty because the implant does not change in thickness even after many years. Thus, we investigated whether changes in implant thickness occurred after rhinoplasty using Surgiform implants.
Methods
This study enrolled 12 patients who had Surgiform nasal implants removed for any reason after receiving rhinoplasty in 2007 or later. After the Surgiform implants were removed, we measured the thickness of the central part of the implants using calipers.
Results
At the time of the initial operation, the mean implant thickness was 4.48┬▒0.30 mm at the supra-tip. At the time of implant removal, the mean thickness was 4.32┬▒0.29 mm. The implants maintained 96.5% of their initial thickness. There was a negligible reduction in the Surgiform implants' thickness over time.
Over the years, a variety of materials have been used for augmentation rhinoplasty. The ideal materials for augmentation are nonallergenic, nontoxic, and noncarcinogenic, and they should produce predictable results with minimal resorption. Autologous or homologous materials such as bone, cartilage, subcutaneous fat, dermis, fascia, and collagen are well-tolerated, but resorption makes their long-term effects unpredictable. Many alloplastic materials, such as silicone, expanded polytetrafluoroethylene (ePTFE), porous high-density polyethylene, filler, and alloplastic rib cartilage have been used to address this issue.
W. L. Gore was the first to use ePTFE in humans; he began to use it in the late 1960s, specifically for vascular grafting. Since then, several million vascular grafts have been performed using ePTFE without any reports of rejection or carcinogenesis [1-3]. The use of ePTFE for nasal augmentation was first introduced in 1983 under the brand name Gore-Tex (W. L. Gore & Associates Inc., Flagstaff, AZ, USA). This implant shows remarkable biocompatibility and excellent tissue integration [4-11]. However, ePTFE has numerous micropores, ranging from 10 to 30 mm in diameter, which increases the risk that it will lose its volume and thickness over time [7]. As the use of ePTFE in augmentation rhinoplasty increases, so does the need for an objective way to evaluate postsurgical outcomes. Multiple clinical studies have reported a postoperative decrease in the thickness of ePTFE implants after rhinoplasty. For this reason, overcorrection became necessary in augmentation rhinoplasty with ePTFE [12-14].
Therefore, we began using another form of soft-type ePTFE, Surgiform (Surgiform Technology, Ltd., Lugoff, SC, USA) as an implant material. Like Gore-Tex, this material comes in sheet form and is flexible. Unlike existing material, Surgiform compensates for the deformation caused by shrinkage, which is a defect of existing Gore-Tex series products. We hypothesized that the physical characteristics of Surgiform would result in the maintenance of its initial thickness, and investigated postoperative changes in the thickness of Surgiform implants.
This study targeted patients who underwent rhinoplasty with a Surgiform implant at the Department of Plastic Surgery at the Nose Aesthetic Plastic Surgery Clinic (Dr. Rho Aesthetic Plastic Surgery) and experienced complications associated with alloplastic materials in 2007 or later. Patients whose Surgiform implant was removed due to infection or inflammation were excluded from the study, but patients who desired reoperation due to aesthetic dissatisfaction were included. All subjects came to our department for revisional rhinoplasty after augmentation rhinoplasty with alloplastic materials. Their clinical characteristics were analyzed retrospectively, including the type of alloplastic materials used and the pattern of complications as assessed by their medical charts and an analysis of their photographs, and histopathologic studies. All authors have read the Declaration of Helsinki and have followed the guidelines in this study.
All surgical procedures were performed by a single surgeon (Dr. Rho) under general anesthesia. An external rhinoplasty approach was used in all cases. An inverted V-shaped incision was made in the mid-columellar area and the subsuperficial muscular aponeurotic system plane was dissected to expose the bony and cartilaginous framework. Subperiosteal dissection was performed from the bony dorsum to the nasion to create a tunnel, and the existing implant was removed via subperiosteal dissection. Extra care was taken to avoid supraperiosteal dissection that might cause postoperative implant instability. A Surgiform implant (Surgiform Technology, Ltd.) was used in all patients. Before inserting the designed graft, the subsuperficial muscular aponeurotic system and subperiosteal tunnel were vigorously irrigated with normal saline to remove blood clots, tissue debris, and possible pathogens. The postoperative care methods did not differ among the enrolled patients.
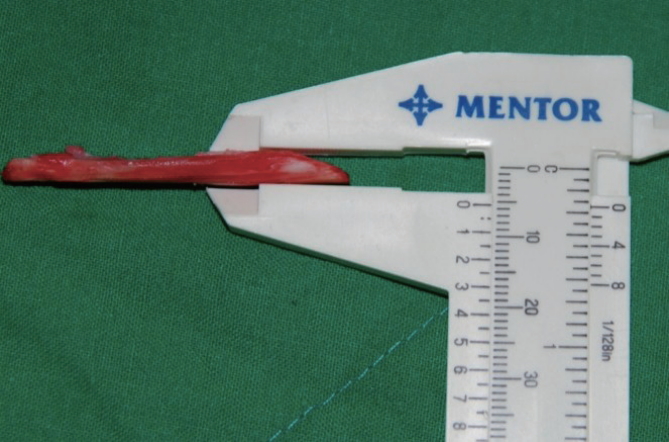
The preoperative initial thicknesses of the Surgiform implants were collected via chart review. Thickness measurements were only made of samples with well-preserved original forms and without extension, tears, or crumpling. After the implants were removed, we measured the thickness of the central part of the implant using Mentor calipers (Fig. 1).
Statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). Initial and follow-up implant thicknesses were compared using the paired t-test. The t-test was used to evaluate correlations according to the follow-up duration (<24 months or Ōēź24 months). The patientsŌĆÖ age, gender, and personal variations in changes in implant thickness showed no significant correlations in our study.
This study group included 12 patients (4 men and 8 women). The patients ranged in age from 20 to 34 years (mean, 24.8 years). The mean time interval between augmentation rhinoplasty and implant removal was 22.25 months. The shortest follow-up period was 7 months, and the longest was 47 months. The patientsŌĆÖ characteristics are summarized in Table 1.
There was a negligible reduction in Surgiform thickness after augmentation rhinoplasty. At the time of the initial operation, the mean implant thickness was 4.48┬▒0.30 mm. At the time of implant removal, the thickness was 4.32┬▒0.29 mm. The implants maintained 96.5% of their initial thickness (Table 2). The P-value was 0.002, indicating a significant difference between the 2 groups, but the amount of reduction was only 0.16 mm (3.5%), which is a negligible decrease compared to previous studies of Gore-Tex implants.
We investigated whether the maintenance of implant thickness depended on the length of the follow-up period. When the follow-up period was <24 months, the initial thickness was 4.61┬▒0.42 mm and the thickness at implant removal was 4.41┬▒0.41 mm, corresponding to 95.9% of the initial thickness. When the follow-up duration was Ōēź24 months, the initial thickness was 4.30┬▒0.46 mm and the thickness at implant removal was 4.19┬▒0.45 mm, corresponding to 97.4% of the initial thickness. These 2 groups were compared using the t-test, and the resulting P-value was 0.417, indicating that duration was not significantly associated with the reduction of Surgiform thickness.
Several approaches to rhinoplasty are currently in use. The most common type of rhinoplasty is augmentation rhinoplasty. Augmentation can be performed with autologous or alloplastic materials. Autologous cartilage is the most widely used and preferred graft material; it remains the gold standard against which other materials are compared [1,12-14]. Autologous materials commonly incorporate well into the surrounding tissues, enabling durable results and the opportunity to replace ŌĆ£like tissue with like tissueŌĆØ [15]. Autologous septal cartilage and auricular concha cartilage are the most commonly selected graft materials in augmentation rhinoplasty. However, in graft-depleted patients and patients with a severely deficient dorsa, costal cartilage and bone or iliac crest bone can be considered [16-18]. Cartilage and bone possess the rigidity needed to maintain major nasal shape changes against the skin envelope and intranasal lining [1,15]. Infections of autologous cartilage grafts are rare, but resorption, displacement, curling, and sharp edges can develop over time [19,20]. Although autologous materials are excellent in terms of infection resistance, issues with biocompatibility and resorption make their long-term effects unpredictable [13,14,21-23].
Worldwide, silicone is the most commonly used implant material for nasal augmentation, especially in Asia [1,8]. Silicone is practically inert, eliciting very little tissue reaction [24,25]. Its firm consistency permits easy sculpting, but can feel like a foreign body under the skin [19,20]. Its surface is thin, which makes handling difficult [19]. Its nonporous structure impedes bacterial colonization, but also prevents tissue ingrowth and biointegration [8,26]. Instead, a thick fibrous capsule surrounds the implant. Implant stabilization depends upon the formation of this capsule, but the capsule itself can predispose the patient to implant malposition and the deformation of overlying tissue. The capsule acts as a nidus for bacterial infiltration, but also serves as a barrier for antibiotic penetration. The widespread use of silicone has been limited by several complications, including inflammation, migration, exposure, calcification, resorption of underlying bone, and abnormal skin color. Perhaps most disconcerting is the tendency for silicone to extrude over time [1,24].
Through the end of 2006, ePTFE (or Gore-Tex) was considered perhaps the most reliable solid implant material available. The manufacturer recently discontinued the fabrication of this material for applications in the field of plastic surgery. However, Gore-Tex sheets for use in general surgery and vascular surgery remain in production. ePTFE is a polymer of carbon bound to fluorine, and is composed of solid pillar-shaped nodes connected by very fine fibrils in a grid pattern. It has the advantages of low tissue reactivity, outstanding biocompatibility, reasonable cost, and a long history of successful use [21]. Bacterial adherence is inhibited by the hydrophobic composition of ePTFE [27]. Its microporous composition encourages tissue ingrowth that promotes implant stability. Its greatest advantage over other alloplasts may be its ability to adhere to surrounding tissues firmly enough to prevent migration, but loosely enough to permit easy retrieval if necessary [21,28]. ePTFE can be easily shaped and exhibits no tendency to undergo resorption, although it may slowly change shape and develop prominent edges over time [21]. Delayed infection or immune reactivity is associated with a short-term extrusion rate of ~3% [29]. Similarly to silicone, ePTFE tends to be slippery, causing possible displacement in the early postoperative period before tissue ingrowth has occurred. The whitish color of the material may become visible externally, particularly in thin-skinned individuals [19].
ePTFE Gore-Tex implants, which were previously widely used, had problems maintaining their initial thickness. Several studies found that the height of ePTFE can decrease during the postoperative period [5,21,28]. Therefore, many plastic surgeons have used non-absorbable materials in order to maintain the shape of the nose and minimize changes in implant thickness [13]. A previous study reported that Gore-Tex lost 29% of its initial height based on objective measurements made using ultrasonography [30].
The thickness of the Surgiform implants did not decrease significantly after augmentation rhinoplasty. We were not sure why there was a difference in the thickness change between Gore-Tex and Surgiform, but we did notice that Surgiform was firmer to the touch. Therefore, we requested information from the manufacturer of Surgiform, and found that Surgiform is 1.5 times denser than Gore-Tex and that the manufacturer treated the surface of Surgiform grafts to prevent excessive tissue ingrowth and capsule formation. Based on this information, we suggest that the extrinsic force exerted by the skin envelope over time was not enough to compress the empty space within the Surgiform material, which might account for this difference between Gore-Tex and Surgiform.
Although a statistically significant difference was found between the thickness of the graft at the time of insertion and the thickness measured during follow-up, the reduction of thickness was only 3.5%, which is negligible compared to previous studies using Gore-Tex implants. Jang et al. [14] examined changes in the thickness in 34 removed Gore-Tex samples. The longer the Gore-Tex samples remained inserted, the more their thickness decreased: from 100% to 73.3% (mean, 86.7%) of normal in 10 samples that were up to 6 months old, from 90% to 50% of normal (mean, 74.5%) in 10 samples that were 7 to 24 months old, from 83% to 50% (mean, 59.3%) in 8 samples that were 25 to 48 months old, and from 60% to 43.3% (mean, 51.7%) of normal in 6 samples that were 49 months or older. In our study, duration did not affect reduction in Surgiform thickness.
Although Surgiform is a relatively safe graft material for augmentation rhinoplasty, surgeons should make every effort to reduce complications. A no-hands technique should be used to manipulate implant materials, and the tissue pocket should be thoroughly irrigated before implant insertion. All rhinoplasties were performed by a single surgeon and the same technique was used to manipulate the Surgiform graft in all patients. These measures were implemented to minimize bias and error.
Our study had limitations. First, the duration of follow-up varied. However, few studies have reported comparable follow-up durations. Second, our study did not include histologic evaluations. Third, our study had a small number of patients. Despite these limitations, it is significant that this study was carried out with samples that were directly removed.
Multiple clinical studies have reported a postoperative decrease in the thickness of Gore-Tex implants after rhinoplasty. Surgiform is another form of soft-type ePTFE that addresses the defects of Gore-Tex implants. Surgiform has the advantage of maintaining its initial thickness and producing a natural nose shape after augmentation rhinoplasty. Thus, Surgiform is more useful for nasal implants than other Gore-Tex implants.
Fig.┬Ā1.
Clinical view of measurements of the thickness of Surgiform implants: the thickest portion was measured.
Table┬Ā1.
Characteristics of 12 patients who underwent augmentation rhinoplasty with Surgiform
| Patient no. | Age | Sex | Initial thickness (mm) | Duration (months) | F/U thickness (mm) | F/U thickness (%)a) |
|---|---|---|---|---|---|---|
| 1 | 20 | F | 5.5 | 47 | 5.3 | 96.36 |
| 2 | 27 | F | 3.0 | 42 | 3.0 | 100.00 |
| 3 | 27 | F | 3.5 | 29 | 3.3 | 92.86 |
| 4 | 20 | M | 5.0 | 26 | 4.9 | 98.00 |
| 5 | 23 | F | 4.5 | 24 | 4.5 | 100.00 |
| 6 | 20 | F | 4.0 | 23 | 3.8 | 93.75 |
| 7 | 29 | M | 4.5 | 20 | 4.1 | 91.11 |
| 8 | 28 | F | 3.0 | 19 | 3.0 | 100.00 |
| 9 | 20 | F | 4.0 | 14 | 3.8 | 95.00 |
| 10 | 22 | M | 5.3 | 8 | 5.3 | 100.00 |
| 11 | 27 | M | 5.0 | 8 | 4.8 | 96.00 |
| 12 | 34 | F | 6.5 | 7 | 6.2 | 95.38 |
Table┬Ā2.
Thickness of the Surgiform according to the insertion duration
| Initial thickness (mm) | Follow-up thickness (mm) | Differencea) (mm) | P-value | Follow-up, % (95% CI) | |
|---|---|---|---|---|---|
| All | 4.48 ┬▒ 0.30 | 4.32 ┬▒ 0.29 | 0.16 ┬▒ 0.04 | 0.002* | 96.5 (94.6-98.5) |
| Duration | 0.417** | ||||
| ŌĆā<24 months | 4.61 ┬▒ 0.42 | 4.41 ┬▒ 0.41 | 0.19 ┬▒ 0.06 | 0.014* | 95.9 (92.9-98.9) |
| ŌĆāŌēź24 months | 4.30 ┬▒ 0.46 | 4.19 ┬▒ 0.45 | 0.11 ┬▒ 0.05 | 0.097* | 97.4 (93.7-100.0) |
REFERENCES
1. Deva AK, Merten S, Chang L. Silicone in nasal augmentation rhinoplasty: a decade of clinical experience. Plast Reconstr Surg 1998;102:1230-7.


2. Zeng Y, Wu W, Yu H, et al. Silicone implant in augmentation rhinoplasty. Ann Plast Surg 2002;49:495-9.


3. Moon KM, Cho G, Sung HM, et al. Nasal anthropometry on facial computed tomography scans for rhinoplasty in Koreans. Arch Plast Surg 2013;40:610-5.



6. Jang YJ, Wang JH, Sinha V, et al. Tutoplast-processed fascia lata for dorsal augmentation in rhinoplasty. Otolaryngol Head Neck Surg 2007;137:88-92.


7. Peled ZM, Warren AG, Johnston P, et al. The use of alloplastic materials in rhinoplasty surgery: a meta-analysis. Plast Reconstr Surg 2008;121:85e-92e.


8. Graham BS, Thiringer JK, Barrett TL. Nasal tip ulceration from infection and extrusion of a nasal alloplastic implant. J Am Acad Dermatol 2001;44:362-4.


9. Pak MW, Chan ES, van Hasselt CA. Late complications of nasal augmentation using silicone implants. J Laryngol Otol 1998;112:1074-7.


10. Erlich MA, Parhiscar A. Nasal dorsal augmentation with silicone implants. Facial Plast Surg 2003;19:325-30.



11. McCurdy JA Jr. The Asian nose: augmentation rhinoplasty with L-shaped silicone implants. Facial Plast Surg 2002;18:245-52.



12. Mendelsohn M, Dunlop G. Gore-tex augmentation grafting in rhinoplasty--is it safe? J Otolaryngol 1998;27:337-41.

13. Jin HR, Lee JY, Yeon JY, et al. A multicenter evaluation of the safety of Gore-Tex as an implant in Asian rhinoplasty. Am J Rhinol 2006;20:615-9.


14. Jang TY, Choi JY, Jung DH, et al. Histologic study of Gore-Tex removed after rhinoplasty. Laryngoscope 2009;119:620-7.


15. Gurley JM, Pilgram T, Perlyn CA, et al. Long-term outcome of autogenous rib graft nasal reconstruction. Plast Reconstr Surg 2001;108:1895. -905. discussion 906-7.


16. Sherris DA, Kern EB. The versatile autogenous rib graft in septorhinoplasty. Am J Rhinol 1998;12:221-7.


17. Gryskiewicz JM. Waste not, want not: the use of AlloDerm in secondary rhinoplasty. Plast Reconstr Surg 2005;116:1999-2004.


18. Celik M, Halilo─¤lu T, Bay├¦in N. Bone chips and diced cartilage: an anatomically adopted graft for the nasal dorsum. Aesthetic Plast Surg 2004;28:8-12.



19. Fanous N, Samaha M, Yoskovitch A. Dacron implants in rhinoplasty: a review of 136 cases of tip and dorsum implants. Arch Facial Plast Surg 2002;4:149-56.



20. Ahn J, Honrado C, Horn C. Combined silicone and cartilage implants: augmentation rhinoplasty in Asian patients. Arch Facial Plast Surg 2004;6:120-3.


21. Godin MS, Waldman SR, Johnson CM Jr. Nasal augmentation using Gore-Tex. A 10-year experience. Arch Facial Plast Surg 1999;1:118. -21. discussion 22.


22. Conrad K, Gillman G. A 6-year experience with the use of expanded polytetrafluoroethylene in rhinoplasty. Plast Reconstr Surg 1998;101:1675. -83. discussion 84.


23. Yang SJ, Lee JH, Tark MS. Problems of expanded polytetrafluoroethylene (Gore-Tex(R)) in augmentation rhinoplasty. J Korean Soc Plast Reconstr Surg 2004;31:28-33.
24. Yanaga H, Koga M, Imai K, et al. Clinical application of biotechnically cultured autologous chondrocytes as novel graft material for nasal augmentation. Aesthetic Plast Surg 2004;28:212-21.


25. Adams JS. Grafts and implants in nasal and chin augmentation. A rational approach to material selection. Otolaryngol Clin North Am 1987;20:913-30.


27. Lovice DB, Mingrone MD, Toriumi DM. Grafts amd implants in rhinoplasty and nasal reconstruction. Otolaryngol Clin North Am 1999;32:113-41.


28. Rothstein SG, Jacobs JB. The use of Gore-Tex implants in nasal augmentation operations. Entechnology 1989:40;40(2): 4-5.






