|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 28(3); 2022 > Article |
|
Abstract
Serratia marcescens is a Gram-negative, facultatively anaerobic bacillus that has been implicated in hospital-acquired infections. Because no previous cases of delayed infections caused by S. marcescens after autologous fat injection have been reported, we introduce a case report. A 74-year-old woman underwent fat injection for aesthetic purposes and visited our hospital for left cheek swelling after this procedure. Blood tests showed a slightly elevated white blood cell count. Facial computed tomography demonstrated an abscess and emergency surgery was performed. A work-up of the necrotic tissue and drained abscess contents was conducted. Cultures showed growth of S. marcescens. Based on the culture results , a proper antibiotic was prescribed. Follow-up blood tests showed normal findings, and there was no recurrent infection or inflammation. In most acute infections after a fat graft, Staphylococcus aureus or Staphylococcus epidermidis can be suspected, while mycobacterial infections are often suspected in cases of delayed infection and chronic inflammation. However, clinicians should keep in mind that there may be infections of uncommon bacteria. When an atypical delayed infection is suspected after an autologous fat graft, it is important to perform aseptic wound culture and biopsy as soon as possible, use appropriate antibiotics, and conduct proper surgical treatment.
Serratia marcescens, which was discovered by Bartolomeo Bizio in 1819, is a Gram-negative motile bacillus belonging to the Enterobacteriaceae family [1]. S. marcescens is a facultative anaerobic bacterium that has been implicated in hospital-acquired infections, particularly catheter-associated bloodstream infections, lower respiratory tract infections, urinary tract infections, and wound infections [2]. Immunocompromised patients are particularly susceptible to infection with S. marcescens. A recent surveillance program in the United States and Europe found that S. marcescens accounted for an average of 6.5% of all Gram-negative infections in intensive care units (ICUs) and ranked fifth among Gram-negative organisms in ICUs, with an especially high frequency in catheter-associated urinary tract and bloodstream infections [3]. This microorganism gives rise to a wide range of infections and is also a common cause of ocular infections, ranging from asymptomatic colonization to keratitis or conjunctivitis [4]. S. marcescens is also a rare cause of endocarditis and soft tissue infections [5], but cutaneous lesions caused by this bacterium are uncommon and few cases of cutaneous S. marcescens infections have been reported in the literature [6].
In cases of unhealed cellulitis more than 2 weeks after a fat graft, the possibility of delayed infection should be suspected. The term “delayed infection” refers to an infection that is not observed in the immediate postoperative period, with an onset between 2 and 10 weeks after surgery. Delayed infections are typically due to less virulent bacteria, and as the duration of infection extends, biofilms mature and become more resistant to antibiotic therapy and host defenses [7].
In this study, we report the case of a 74-year-old patient who developed delayed facial cellulitis due to S. marcescens after a fat graft was performed for aesthetic purposes.
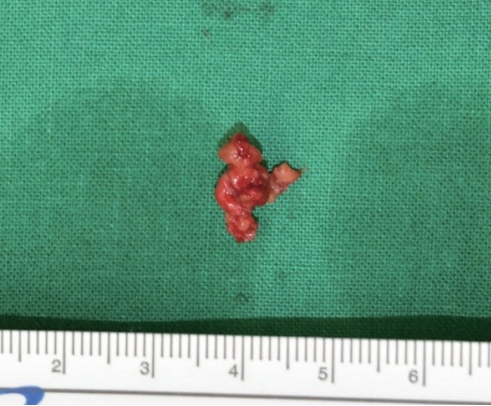
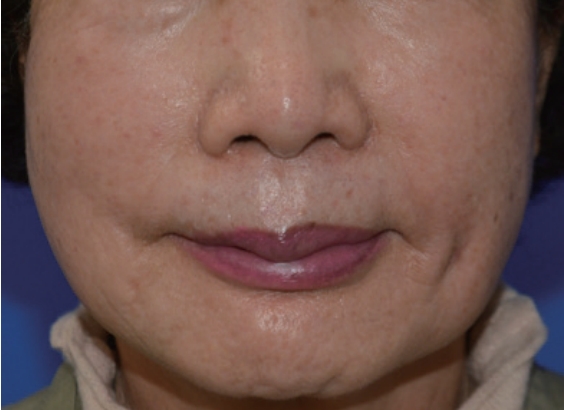
A 74-year-old woman with no specific medical history underwent autologous fat injection for aesthetic purposes at a plastic surgery clinic and visited our hospital for left cheek swelling after this procedure. The patient had undergone cosmetic fat grafting several times, and in response to the unsatisfactory results of the left nasolabial fold, she received a fat regraft 4 weeks before symptom onset. The patient initially experienced a slight fever accompanied by edema, erythema, firmness, serous discharge, and an uncomfortable sensation in the injected site. Pain and foul odor were absent. She had received antibiotic treatment and a steroid injection because of a hard mass-like lesion, thought to be a granuloma, but it did not improve. She visited our hospital approximately 4 months after undergoing surgery at the plastic surgery clinic, and the swelling and erythema in her left cheek had worsened by the time of her initial visit to our hospital (Fig. 1). She had good oral hygiene and did not have any other infections such as sinusitis. Upon admission, she did not have a fever and had a C-reactive protein level and erythrocyte sedimentation rate in the normal range (0.5 mg/dL and 14 mm/hr, respectively), but blood tests showed an elevated white blood cell (WBC) count of 12,600 cells/μL. Contrast-enhanced facial computed tomography demonstrated a marked 2.2×2.4-cm abscess within the left upper mandibular vestibule, and emergency surgery was performed using a submalar horizontal incision about 1 cm in length to remove the unhealthy tissue (Figs. 2, 3). The abscess was drained using a silicone drain. The necrotic tissue and drained abscess contents were analyzed for the presence of Gram-positive bacteria, Gram-negative bacteria, anaerobic bacteria, fungi, and mycobacteria by performing a work-up including a tuberculosis/non-tuberculous mycobacteria (TB/NTM) polymerase chain reaction (PCR) assay and cultures.
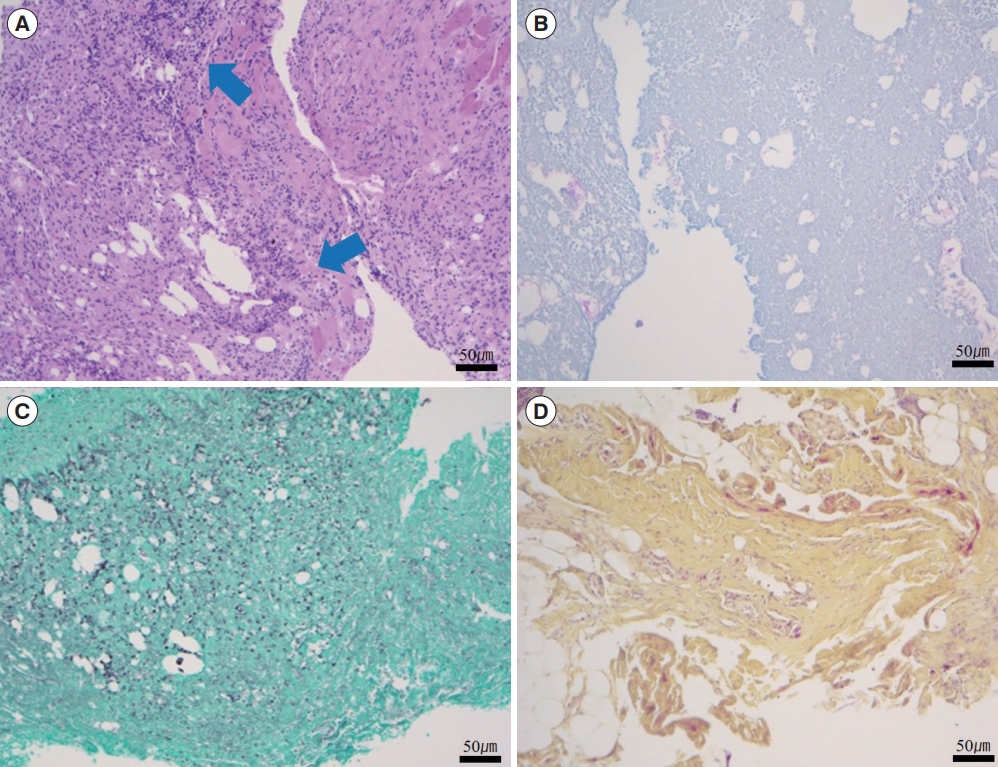
The infected tissue biopsy (with hematoxylin and eosin staining) showed acute interstitial inflammation with deeply infiltrated inflammatory cells (Fig. 4). Acid-fast bacillus (AFB) staining, Gomori methenamine silver staining, and Gram-positive culture were all negative. However, cultures from the tissue and drained abscess showed growth of S. marcescens. During the interval after the patient had been admitted and before wound cultures had been performed, an infection caused by resident skin flora such as Staphylococcus aureus was assumed, and empiric first-generation cephalosporin (1 g intravenous twice daily) was administered while waiting for the culture results and a consultation from an infectious disease specialist. Based on the results of cultures and antimicrobial susceptibility testing (AST), a second antibiotic (fourth-generation cephalosporin, 2 g intravenous once daily) was prescribed. Repeated blood cultures showed a normal WBC count (5,080 cells/μL). The patient’s condition improved, and antibiotics were changed from intravenous to oral (fluoroquinolone, 250 mg twice daily) based on the consultation with the infectious disease specialist (Table 1). She received antibiotic treatment for 6 weeks, after which a follow-up blood test showed normal findings. The patient recovered fully and was satisfied with the results. The scar was managed with pulsed dye laser and ablative CO2 fractional laser treatment. No complications, such as recurrent infection or inflammation, occurred (Fig. 5).
S. marcescens, a Gram-negative member of the Enterobacteriaceae family belonging to the Serratia genus, was previously thought to be a harmless bacterium abundantly present in the environment [8]. This opportunistic pathogen came to be recognized as a cause of human infections approximately 30 years ago based on several nosocomial outbreaks. S. marcescens commonly causes nosocomial infections, especially in immunocompromised hosts and in the ICU setting [9]. Special attention needs to be paid because community-acquired infections are rare, but outbreaks of nosocomial infections are often reported in children or adults. In recent years, numerous cases and articles have described bacterial infections after fat grafts [10]. If there is a clinical suspicion of an NTM infection or another unusual infection in patients with chronic infections and inflammation after a fat graft, an appropriate diagnosis and treatment plan should be established [10].
Delayed nodule formation after an autologous fat graft can occur due to various causes, including fat necrosis, cyst formation, or an unusual delayed infection. When there is an unhealed infection more than 2 weeks after a fat graft, the possibility of a delayed infection should be suspected. Delayed infections are typically exogenously acquired and mainly caused by low-virulent microbes such as coagulase-negative staphylococci [8,11].
The author previously experienced several delayed infections, such as NTM infections of surgical wounds in patients who had been treated at plastic surgery clinics. After the fat regraft, the patient presented herein received a steroid injection and then experienced recurrent soft tissue inflammation with a waxing and waning course. Since a mycobacterial infection was suspected, AFB staining and a TB/NTM PCR test for Mycobacterium tuberculosis and NTM were conducted, as well as Gram staining and a routine culture test. The results of AFB staining and TB/NTM PCR were negative, whereas S. marcescens was confirmed in tissue culture. The patient was hospitalized to receive antibiotic therapy based on AST of cultures and a consultation with an infectious disease specialist. Symptoms such as swelling and tenderness started to resolve approximately 3 weeks after treatment initiation. During the 6-week monitoring period thereafter, there were no complications or recurrent symptoms. A hard mass-like lesion that was initially present along part of the wound margin was not palpable.
From an aesthetic standpoint, autologous fat is considered an ideal material from an aesthetic standpoint for expanding tissue because, unlike artificial materials and fillers, it is not associated with hypersensitivity or foreign body reactions [12]. However, the multiple processes required for autologous fat transplantation take time and may lead to complications such as pain, bruises and asymmetric or irregular contours in contrast to procedures using fillers. Nonetheless, autologous fat transplantation has been widely used in the field of aesthetic plastic surgery and reconstruction [12].
In general, an infection that occurs after autologous fat injection is considered to be a negative result that is mostly attributable to unhygienic instruments, improper surgical technique, the patient’s poor skin preparation, and problems with postoperative wound management [13]. Additionally, we speculate that the air conditioning (AC) duct or ventilation system site could serve as a reservoir of nosocomial pathogens in the operating room of a community hospital. The AC and ventilation-control system in local clinics is often operated centrally and poorly managed. Contaminated airflow to the operating room is delivered through large air ducts, which are fitted with contaminated microbial air filters [11]. Therefore, we suggest that airborne transmission may be a source of nosocomial infections during fat graft procedures. In particular, the risk of contamination is higher when using cryopreserved fat than when using fresh fat. Cryopreserved fat contains a low number of viable cells, which leads to a high risk of fat necrosis or inflammation or infection in the graft site [10]. Fresh fat can be contaminated during processes such as fat harvest and centrifugation, but the additional processes required for using cryopreserved fat (e.g., freezing, storage, and thawing) can also cause contamination. Stored fat may be contaminated during cryopreservation, and procedures using contaminated fat lead to wound infection [14].
Once an infection has occurred in transplanted fat, it is important to remove infected and necrotic tissue as soon as possible, conduct AST, and administer empirical antibiotics until the AST results become available [10]. In such situations, it is difficult for surgeons to make aggressive decisions to perform surgical treatments such as incision and drainage in patients who have received cosmetic procedures. Therefore, if an infection occurs after an autologous fat graft, it is important to diagnose the infection rapidly in its early stages, conduct all possible sensitivity tests before treatment, and in case of treatment failure or after recurrence, select appropriate antibiotics based on AST results. Empirical antibiotics can be administered until the results of AST become available.
In most acute infections after an autologous fat graft, S. aureus, Staphylococcus epidermidis, or Streptococcus pyogenes can be suspected, while mycobacterial infections are often suspected in cases of delayed infection and chronic inflammation [15]. However, clinicians should keep in mind that there may be infections of uncommon bacteria, as in this case, and they should quickly conduct cultures and perform AST. Empirical antibiotics can be used until the AST results become available, and clinicians should simultaneously perform an additional work-up to diagnose the infection. When an atypical infection is suspected, it is important to perform aseptic wound culture and biopsy in the operating room as quickly as possible, and to conduct an appropriate surgical intervention such as incision and drainage or removal of the inflammatory abscess depending on the location and clinical manifestations of the lesion.
In conclusion, autologous fat grafts have been widely used in reconstructive and aesthetic surgery, and the procedure sometimes causes delayed infections. Because no previous cases of cutaneous delayed infections caused by S. marcescens after autologous fat injection, which is one of the most common aesthetic surgical procedures, have been reported in the literature, the present case report is noteworthy. The general complications associated with this procedure include infections, edema, hematoma, bruises, granuloma, and cyst formation. Sequelae such as fat necrosis, a scar after incision, and asymmetry may lead to unsatisfactory aesthetic outcomes in the long term. Therefore, it is important to manage the cryopreserved fat, maintain the sterility of surgical instruments and perform aseptic treatment procedures and techniques to prevent complications. Additionally, AC ducts or ventilation system sites in the operating room should be kept thoroughly clean to maintain a sterile airflow to avoid airborne transmission. When an atypical infection (e.g., S. marcescens in this case) is suspected, it is important to perform aseptic wound culture and biopsy as soon as possible, use appropriate antibiotics based on the results of AST, and conduct proper surgical treatment.
Notes
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Fig. 2.
Contrast-enhanced facial computed tomography demonstrating a marked 2.2×2.4-cm abscess within the left upper mandibular vestibule (red arrow).
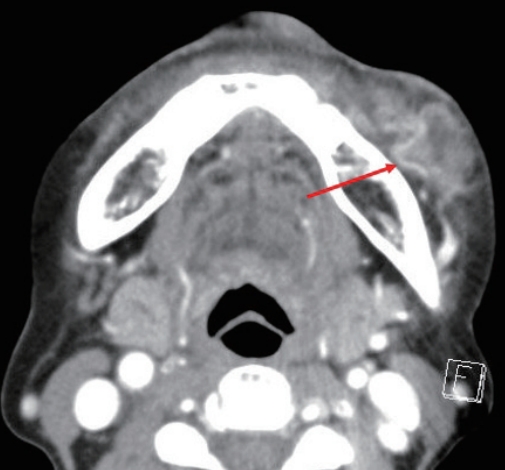
Fig. 4.
Histopathologic appearance of the necrotic tissue. Histopathologic examination showing acute interstitial inflammation with deeply infiltrated inflammatory cells (blue arrows). (A) Hematoxylin and eosin staining. (B) Gram staining. (C) Gomori methenamine silver staining. (D) Acid-fast bacillus staining (×100).
REFERENCES
2. Gupta N, Hocevar SN, Moulton-Meissner HA, et al. Outbreak of Serratia marcescens bloodstream infections in patients receiving parenteral nutrition prepared by a compounding pharmacy. Clin Infect Dis 2014;59:1-8.



3. Sader HS, Farrell DJ, Flamm RK, et al. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis 2014;78:443-8.


4. Atlee WE, Burns RP, Oden M. Serratia marcescens keratoconjunctivitis. Am J Ophthalmol 1970;70:31-3.


5. Mills J, Drew D. Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse. Ann Intern Med 1976;84:29-35.


6. Joao AM, Serrano PN, Cachao MP, et al. Recurrent Serratia marcescens cutaneous infection manifesting as painful nodules and ulcers. J Am Acad Dermatol 2008;58(2 Suppl): S55-7.


7. Chang CH, Chang YY, Lu PH. Non-tuberculous Mycobacteria infection following autologous fat grafting on the face. Aesthet Surg J 2017;38:NP1-5.


8. Eisenstein BI, Zaleznik DF. Enterobacteriaceae. In: Mandell GL, Douglas RG, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 2297-310.
9. Yu VL. Serratia marcescens: historical perspective and clinical review. N Engl J Med 1979;300:887-93.


10. Kim SK, Choi JA, Kim MH, et al. Treatment of the Mycobacterium chelonae Infection after Fat Injection. Arch Plast Surg 2015;42:68-72.



11. Uduman SA, Farrukh AS, Nath KN, et al. An outbreak of Serratia marcescens infection in a special-care baby unit of a community hospital in United Arab Emirates: the importance of the air conditioner duct as a nosocomial reservoir. J Hosp Infect 2002;52:175-80.


12. Bucky LP, Kanchwala SK. The role of autologous fat and alternative fillers in the aging face. Plast Reconstr Surg 2007;120(6 Suppl): 89S-97S.


13. Lee BS, Kim TR, Yoo SW, et al. A clinical analysis of nosocomial coagulase negative staphylococcal bacteremia. Korean J Intern Med 1991;40:240-9.