|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 29(1); 2023 > Article |
|
Abstract
An advantage of breast augmentation with injectable fillers is that the desired size can be determined and achieved under local anesthesia with a short recovery time. However, a high complication rate is a critical disadvantage. Some fillers are challenging to remove, resulting in breast deformity and scarring. Five patients who underwent surgery to manage a foreign body in the breasts in 2021 were enrolled in this study. Two had copolyamide filler injections, while the other three had polyacrylamide hydrogel filler injections. A physical examination was performed, and preoperative and intraoperative photographs were obtained. Two patients underwent subcutaneous mastectomy because most of the filler had infiltrated into the normal breast tissue. In contrast, the other patients underwent filler removal and debridement because most of the filler had remained separate from the normal breast tissue. All patients who had a subcutaneous mastectomy and one who underwent only filler removal underwent immediate breast reconstruction with cohesive gel implants. The other patients rejected immediate reconstruction, and only filler removal was performed. All patients recovered without complications. We propose an algorithm for diagnosis and treatment based on our cases, which we hope can help clinicians manage the complications of filler injections for breast augmentation.
Various breast augmentation methods have been proposed. Among them, breast augmentation using filler injections is advantageous insofar as it allows the patient’s desired size to be achieved and can be performed under local anesthesia with a short recovery time [1].
Several types of fillers have been used, including copolyamide, polyalkylimide gel, polyacrylamide gel, polymethylmethacrylate, and hyaluronic acid [2]. Aquafilling (Biomedica) is a non-absorbable hydrophilic gel composed of 98% sodium chloride solution (0.9%) and 2% copolyamide. Injection with Aquafilling effectively corrects unfavorable results after breast augmentation. Single-use procedures with large-volume injections for breast augmentation have been performed worldwide. However, the safety of this product is controversial because post-injection complications have been described in many case reports [3]. Polyacrylamide hydrogel (PAAG) is a non-absorbable filler composed of 2.5% to 5% hydrophilic polyacrylamide and water. It is well known as Amazingel, which was first introduced in the early 1990s and has been widely used in China and Russia as an injection filler for breast augmentation and facial rejuvenation. It was banned in China in 2006; however, more than 30 million women have received PAAG injections for breast augmentation. Many patients with PAAG injections present with complications, such as inflammation, infection, lumps, deformities, a foreign body sensation, systemic toxicity, and immune system changes [4,5].
In this study, we present five cases of previous breast augmentation with filler injections (two with copolyamide filler and three with PAAG filler). We compare the clinical features, radiologic findings, and characteristics of copolyamide and PAAG fillers, and propose an algorithm for diagnosis and treatment based on the case characteristics.
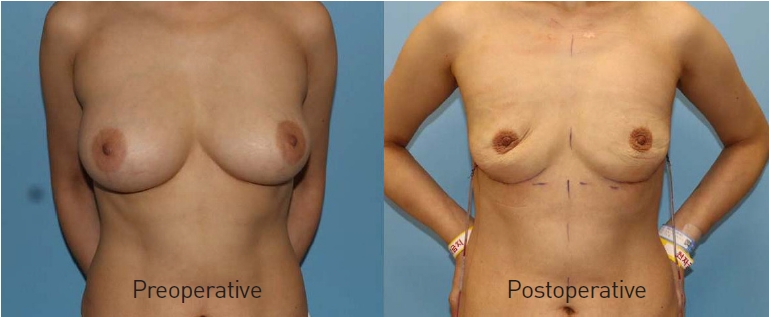
A 47-year-old woman with a history of Aquafilling filler injections in both breasts 7 years ago presented with a mass on her left breast on a regular ultrasound checkup. The mass was identified as an invasive ductal carcinoma on core needle biopsy. Preoperative magnetic resonance imaging (MRI) showed an abnormal mass in the left breast with bilateral filler collection in the subglandular space. There were no specific findings on a physical examination. Most of the filler was separated from the breast tissue and removed from both breasts, and nipple-sparing mastectomy of the left breast, with breast reconstruction using a tissue expander with an acellular dermal matrix, was performed. We removed most of the filler from the right breast after performing a periareolar incision, and the pocket was clean with a fine and thin capsule and no abnormal findings. Massive irrigation and implant insertion were performed in the right breast. The patient recovered without any complications, even after radiation therapy for the left breast.
A 39-year-old woman presented with breast hardening to our outpatient clinic. The patient had received Aquafilling filler injections on both breasts 8 years ago at a local clinic. MRI showed a foreign body and asymmetric fluid collection (Fig. 1A). In addition, hardening of the breast tissue without tenderness was identified on a physical examination. After performing a periareolar incision, subcutaneous mastectomy was performed because most of the filler was attached to the normal breast tissue (Fig. 1B), and 275 cc gel implants (Mentor, MemoryGel) were inserted into the pockets. The specimens weighed 158 g (left) and 142 g (right) (Fig. 1C). Wound healing took place without postoperative complications, and satisfactory aesthetic outcomes were achieved.
With a 20-year history of PAAG filler (Amazingel) injections in both breasts, a 60-year-old woman presented with tenderness in her breasts. Breast MRI showed that the filler had infiltrated into the breast parenchyma without any isolated filler. On physical examination, her intermammary cleft was blunted with abnormal tissue, which was suspected to have been caused by filler migration. Both breasts were firm and hard. The patient requested complete removal of the fillers and a reduction of breast size. The operation was performed with a reduction mammaplasty design, and the fillers showed infiltration to the normal breast tissue without any fluid collection. In total, 612 g (left) and 655 g (right) of the breast tissue-filler complexes were removed. No dehiscence or other wound problems were observed. The patient recovered well and was satisfied with the improvement in her chief complaint.
A 50-year-old woman presented with pain in both breasts for 6 months. She had received PAAG filler (Amazingel) injections for breast augmentation in China 16 years ago. On preoperative MRI, bilateral filler collection in both retromammary fat layers was observed, without other abnormal findings (Fig. 2A). Through an inframammary fold incision, most of the filler was removed with massive irrigation (Fig. 2B and C). Both pockets were cleaned, and there were no abnormal findings in the capsule or adjunct tissues. At the patient’s request, implant-based reconstruction was not performed immediately. The patient recovered well without complications (Fig. 3).
A 59-year-old woman presented with pain and deformity of her breasts for 1 month. The patient had undergone breast augmentation with a PAAG filler (Amazingel) 20 years ago, and surgical removal had already been performed at another hospital. MRI showed filler in the retromammary fat tissue without other lesions. The filler was successfully removed using an inframammary fold incision because it had not infiltrated into the breast tissue. The patient refused immediate reconstruction, and only filler removal was performed. The patient recovered without complications, and an acceptable aesthetic outcome was achieved.
Although the use of fillers for body contouring, including breast augmentation, has not been approved by the Food and Drug Administration, fillers (e.g., hyaluronic acid, copolyamide, and polyacrylamide) have been and are still being used for breast augmentation [4,6,7].
The use of a PAAG filler (Amazingel) was approved by the Chinese Food and Drug Administration; however, it was banned in 2006 because of associated complications. Complications, such as infection, filler migration, lumps, and hematoma, have been recently reported. Breastfeeding issues have also been reported [8], and the filler is suspected of causing breast cancer [9,10]. Jin et al. [10] reported four cases of breast cancer after PAAG filler injections; these patients’ pathologic findings were invasive ductal carcinoma in three cases and lobular carcinoma in one case.
A copolyamide filler, Aquafilling, appears to be a promising new option for replacing PAAG fillers, especially in South Korea [8]. However, according to the Korean Food and Drug Administration document, Aquafilling has a similar composition to PAAG, and the Korean Academic Society of Aesthetic and Reconstructive Breast Surgery expressed serious concerns over Aquafilling injections for breast augmentation in 2016 [11]. Complications after copolyamide filler injection have been identified. Namgoong et al. [8] reported 146 cases of breast and buttock augmentation using a copolyamide filler (Aquafilling); the most common complication was induration and masses, which accounted for 83.6% of 146 cases, followed by pain, firmness, asymmetry, and migration of fillers, with a mean onset of 38.5 months. Nomoto et al. [3] reported that 29 patients who received copolyamide filler injections presented with complications, such as deformity, migration, infection, induration, pain, and fistula; these were complications similar to those of PAAG filler injections.
In our cases, pain, firmness, and enlargement of the breasts were identified with symptom onset occurring 13.2 years post-injection on average. One patient (case 1) showed invasive ductal carcinoma, but the relationship with the filler injection is unclear. Common complications reported in other studies, such as migration, masses, and infection, were not found in our cases, and studies also vary in terms of the interval until symptom onset, which was 13.2 years in our study, but less than 10 years in others [3,5,7,8].
Similar to other studies, our cases showed no significant difference in clinical, radiological, and intraoperative findings between PAAG and copolyamide fillers. Contrary to what was initially reported by the manufacturer and some practitioners [12], there is only a slight difference between these two fillers, and it cannot be said that copolyamide is safer and superior to PAAG fillers in clinical use [3]. Supporting this opinion, a nuclear magnetic resonance analysis of Aquafilling and PAAG fillers showed that the composition of Aquafilling is very similar to that of PAAG fillers [3].
As the complications and risks of filler injection for breast augmentation are widely known, the use of filler injections for breast augmentation has been greatly reduced. However, many patients still have fillers in their breasts because of a fear of breast deformity and size reduction after filler removal [13]. These patients may visit clinics in the future. We propose a management algorithm for these patients based on their clinical manifestations and radiologic findings (Fig. 4). In this algorithm, patients are divided based on several representative clinical manifestations, and different treatment methods can be applied accordingly.
First, after a physical examination and history taking, breast MRI should be performed, and if there is any mass-like lesion on the patient’s MRI, a core needle biopsy should be performed. If the biopsy result is cancer, the patient should undergo surgery for breast cancer first. If the mass is not cancerous or there is no mass on MRI, the presentation of the filler on MRI should be considered, and patients are divided according to whether the filler has infiltrated into the breast tissue group or remains separate from the breast tissue. In the filler infiltration group, subcutaneous mastectomy is performed through an inframammary fold incision or a previous incision scar (with mass excision, if present). Immediately after the procedure, breast reconstruction can be performed according to the patient’s request. In patients with filler that remains separate from the breast tissue, filler removal with debridement and massive irrigation should be performed first. After cleaning all the pockets that contained filler, we check the inside of the pockets and the surrounding capsule. If the pocket is clean with a soft, thin capsule, immediate reconstruction is possible. However, if there is any sign of capsular contracture, total capsulectomy should be performed before reconstruction.
We prefer to insert a cohesive gel implant in a pre-existing subglandular pocket where filler was present if the thickness and circulation of the breast flap are satisfactory. However, if there is a significant skin defect, two-stage reconstruction using a tissue expander or autologous tissue transfer should be considered. In cases with breast cancer, such as case 1 in this study, total mastectomy should be performed first, and the reconstruction method will be determined based on the degree of the skin defect. With a minimal skin defect, direct-to-implant reconstruction can be performed using either the prepectoral or dual-plane method. However, if the skin defect is significant, two-stage reconstruction using a tissue expander or autologous tissue transfer should be considered. If postoperative radiotherapy is planned, we prefer two-stage reconstruction with tissue expander instead of autologous tissue transfer because of concerns about the shrinkage of the transferred tissue after radiation therapy resulting in unpleasing aesthetic outcomes. Since some patients may have negative perspectives regarding reconstruction using breast implants due to their previous experience with fillers, it is necessary to explain and discuss with the patients sufficiently before the operation to determine whether they will opt for reconstruction and, if so, which method of reconstruction will be used.
We hope to provide clinicians with an understanding of the clinical manifestations, radiologic findings, and intraoperative findings of PAAG and copolyamide filler complications and to help initiate the diagnosis and treatment process through this case series and algorithm. An early diagnosis and proper management of these complications will help solve the medical and social problems related to breast augmentation using filler injections.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Soonchunhyang University Hospital (IRB No. 2022-10-016).
Patient consent
The patients provided written informed consent for the publication and use of their images.
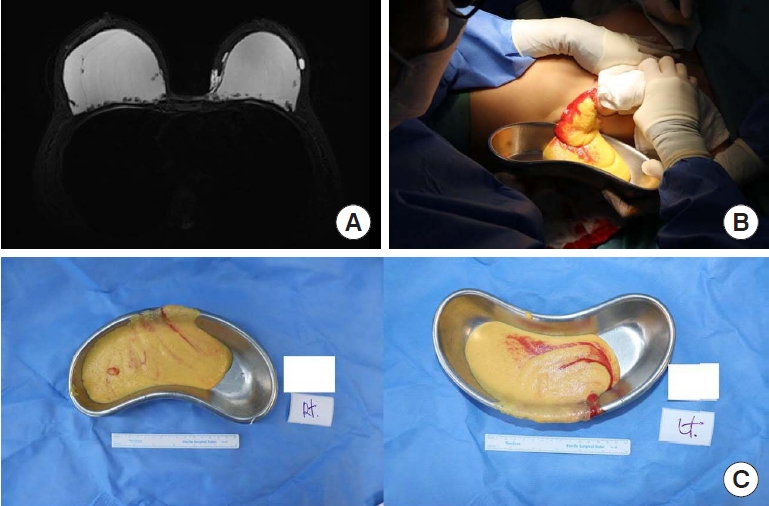
Fig. 1.
Case 2. (A) Most of the injected filler had infiltrated the surrounding tissues on preoperative magnetic resonance imaging. (B) A periareolar incision was performed to open the filler pocket. An intraoperative view shows abnormal breast tissue with infiltrated filler. (C) All the abnormal breast tissue was removed from both breasts.

Fig. 2.
Case 4. (A) The majority of the filler was separate from the normal breast tissue on magnetic resonance imaging. (B, C) It was possible to perform only filler removal, and reconstruction was not carried out. Approximately 150 cc of filler was removed from each breast.
REFERENCES
1. Ishii H, Sakata K. Complications and management of breast enhancement using hyaluronic acid. Plast Surg (Oakv) 2014;22:171-4.




2. Trignano E, Baccari M, Pili N, et al. Complications after breast augmentation with hyaluronic acid: a case report. Gland Surg 2020;9:2193-7.



3. Nomoto S, Hirakawa K, Ogawa R. Safety of copolyamide filler injection for breast augmentation. Plast Reconstr Surg Glob Open 2021;9:e3296.



4. Ebisudani S, Inagawa K, Suzuki Y, et al. Unilateral breast inflation caused by breastfeeding after polyacrylamide hydrogel injection. Plast Reconstr Surg Glob Open 2021;9:e3335.



5. Choi J, Kim YS, Oh DY. Remote migration of breast filler to the inguinal area: a case report. Arch Aesthetic Plast Surg 2021;27:149-52.


6. Hedén P, Sarfati I, Clough K, et al. Safety and efficacy of stabilized hyaluronic acid gel for breast enhancement. Plast Reconstr Surg Glob Open 2016;3:e575.



7. Loesch JM, Eniste YS, Dedes KJ, et al. Complication after Aquafilling® gel-mediated augmentation mammoplasty: galactocele formation in a lactating woman: a case report and review of literature. Eur J Plast Surg 2022;45:515-20.


8. Namgoong S, Kim HK, Hwang Y, et al. Clinical experience with treatment of Aquafilling filler-associated complications: a retrospective study of 146 cases. Aesthetic Plast Surg 2020;44:1997-2007.



9. Cheng NX, Liu LG, Hui L, et al. Breast cancer following augmentation mammaplasty with polyacrylamide hydrogel (PAAG) injection. Aesthetic Plast Surg 2009;33:563-9.



10. Jin R, Luo X, Wang X, et al. Complications and treatment strategy after breast augmentation by polyacrylamide hydrogel injection: summary of 10-year clinical experience. Aesthetic Plast Surg 2018;42:402-9.



11. Roh TS. Letter: Position Statement of Korean Academic Society of Aesthetic and Reconstructive Breast Surgery: Concerning the Use of Aquafilling(R) for Breast Augmentation. Arch Aesthetic Plast Surg 2016;22:45-6.