Does the dominant hand’s use affect the complication rates in prosthetic breast reconstruction?
Article information
Abstract
Background
Numerous studies have investigated risk factors for unfavorable outcomes in prosthetic breast reconstruction, such as obesity, perioperative radiotherapy, and acellular dermal matrix use. However, no reports have explored whether the use of the dominant hand influences complications in breast reconstruction. To address this gap in the literature, analyzed complication rates between the dominant and non-dominant sides after reconstruction.
Methods
We retrospectively reviewed the charts of 160 patients (170 breasts) who underwent breast reconstruction from February 2017 to March 2022. We analyzed the complications between beasts on the dominant and non-dominant sides according to the reconstruction method.
Results
During prosthetic breast reconstruction, the drainage volume and duration on the dominant side exceeded those on the non-dominant side after reconstruction (duration: 9.79 days on the dominant side vs. 9.12 days on the non-dominant side, P=0.196; volume: 771.1 mL on the dominant side vs. 654.3 mL on the non-dominant side, P=0.027). The incidence of complications such as wound dehiscence, mastectomy flap necrosis, and infection was significantly higher in the dominant hand group (infection: 6 vs. 0, P=0.014; dehiscence: 15 vs. 4, P=0.009; flap necrosis: 13 vs. 4, P=0.024).
Conclusions
Complications including seroma, infection, and mastectomy skin flap necrosis following prosthetic reconstruction were common in breasts on the dominant-hand side. Therefore, meticulous management and restriction of shoulder movement can aid in preventing seroma-related complications in prosthetic breast reconstruction, especially on the side of the dominant hand.
INTRODUCTION
Breast reconstruction following mastectomy can be accomplished through two primary surgical techniques: the first involves the use of prosthetics such as tissue expanders and silicone implants, while the second utilizes autologous flap reconstruction. As prosthetic breast reconstruction grows in popularity, it becomes increasingly important to predict and prevent complications related to the prosthesis. Numerous studies have identified risk factors for complications in prosthetic breast reconstruction, including obesity, perioperative radiotherapy, and the use of acellular dermal matrix (ADM) [1]. Infection is a serious complication of prosthetic breast reconstruction. It can be caused by seroma formation, which can also lead to implant failure or serve as an indicator of poor wound healing and hidden infection.
The occurrence of seroma following prosthetic breast reconstruction can range between 0.2% and 20% [1-6]. There is a strong correlation between seroma and the volume of postoperative drainage. The use of a closed suction drain can help prevent the formation of seromas. However, keeping the drain in for an extended period can increase the risk of infection. The exact cause of seroma formation remains unclear [7], but several studies have proposed potential factors in patients who have undergone mastectomy. These include the creation of a large and irregular dead space due to the mastectomy, the insertion of a foreign body (such as a tissue expander, implant, or ADM), movement of the chest wall, and disruption of the lymphatic system [8-11].
In our experience, seromas and extended periods of drain maintenance are more frequently observed in the reconstructed breasts on the side of a patient’s dominant hand. We hypothesized that the inevitable use of a patient’s dominant hand would increase shoulder and pectoralis muscle mobility, potentially contributing to an increase in drainage fluid and the occurrence of acute complications.
To the best of our knowledge, no prior study has explored the degree to which the use of the dominant hand affects complications in breast reconstruction. Therefore, this study aimed to analyze the complications in dominant and non-dominant hand-side breasts following reconstruction.
METHODS
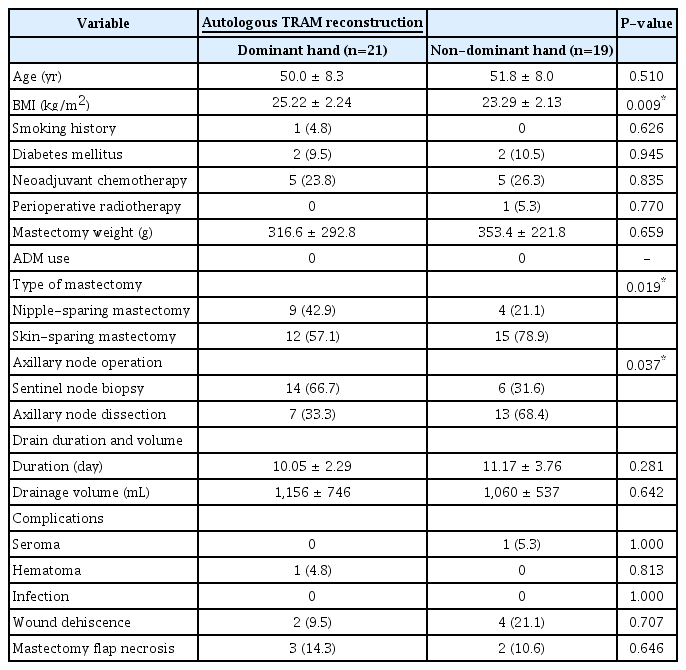
This retrospective cohort study received approval from the Institutional Review Board (IRB No. 2022-11-051). We conducted a retrospective review of 160 patients (170 breasts, including 10 bilateral cases) who underwent prosthetic breast reconstruction at the Bundang CHA Medical Center between February 2017 and March 2022. We divided the patients into two groups according to whether the reconstructed breast was on their dominant or non-dominant hand. During the same period, we also analyzed 40 breasts that underwent autologous transverse rectus abdominis musculocutaneous (TRAM) reconstruction for comparison with the prosthetic reconstruction. Patients who underwent prosthetic breast reconstruction were not restricted in their movement, but they were advised to limit movement of the arm on the same side as the reconstructed breast until all drains were removed. Conversely, patients who underwent TRAM reconstruction were advised to avoid moving the arm on the same side that underwent surgery and were bedridden for 3 to 5 days postoperatively to prevent complications at the donor site.
Patient data including age, body mass index (BMI), diabetes mellitus, smoking history, resected breast tissue weight (g), surgical information, preoperative chemotherapy, and radiotherapy were collected from their medical records. The complications, drainage volume, and drain maintenance period of the patients according to whether surgery was performed on the dominant or non-dominant hand side.
Two plastic surgeons carried out immediate breast reconstruction following mastectomy, which was performed by four breast cancer surgeons. They also conducted delayed breast reconstruction. The choice between single-stage and two-stage reconstructions using a tissue expander was determined by the type of mastectomy and the condition of the flap, as assessed by the plastic surgeons. The implants were placed either in a dual plane (between the pectoralis major muscle and the ADM) or in the prepectoral plane. The silicone implants used were either Mentor smooth round implants or BellaGel smooth-fine implants. In cases where patients underwent two-stage breast reconstruction, tissue expanders (Mentor Corp.) were inserted into the breast pocket. Three types of ADMs were utilized: Alloderm (LifeCell Corp.), Megaderm (L&C BIO Inc.), and CG CryoDERM (CGBio Corp.). The breast and axillary pockets were connected. One drain was placed in the axillary area, and the other was positioned at the lower pole of the breast. The total drainage volume was calculated by adding the volumes from both drains. The closed suction drain was removed when the drainage volume fell below 30 mL for two consecutive days.
The collected data were analyzed using SPSS version 22 (IBM Corp.). Descriptive statistics were used to summarize patient characteristics and surgical details. Continuous variables were presented as means with standard deviations. Categorical variables were reported as frequencies and percentages. In all statistical comparisons, a P-value < 0.05 was considered a significant difference between the two groups (dominant hand vs. non-dominant hand).
RESULTS
In total, 160 patients (170 breasts) who underwent breast reconstruction with prosthetics were analyzed in this study. Ten patients underwent bilateral breast reconstruction. Among the 160 patients, six were left-handed but used their right hand exclusively for writing.
Table 1 presents the demographic characteristics of patients who enrolled in the study. There were no significant disparities in factors that could influence seroma formation and other complications between the dominant hand and non-dominant hand groups. ADMs were utilized to cover either the entire or a portion of the prosthetics in both groups. The mean age of patients in the dominant hand group was 49.1 years (with a standard deviation of 8.3 years), while in the non-dominant hand group, it was 48.1 years (with a standard deviation of ±8.4 years). The mean BMI was within the normal range, with no significant differences observed between the two groups (dominant: 23.79 ± 3.06 kg/m2 vs. non-dominant: 22.94 ± 5.14 kg/m2). Of the six left-handed patients, three who underwent left breast reconstruction were categorized in the dominant hand group, while the rest were placed in the non-dominant hand group.
The mean amount of resected breast tissue was not significantly different between the two groups. In the dominant hand group, it was 354.7 g (range, 95.5–966.0 g). In the non-dominant hand side group, the amount of resected tissue was 313.9 g (range, 90.0–841.0 g). The rate of axillary node dissection was higher in the dominant hand group (26.7%) than in the non-dominant hand group (17.9%), but the difference was not statistically significant (P = 0.378).
In the prosthetic breast reconstruction group, the drainage volume in the dominant hand group was significantly larger than that in the non-dominant hand group (volume: 771.1 mL on the dominant side vs. 654.3 mL on the non-dominant side, P = 0.027). Although there was no statistically significant difference in the mean drain maintenance period, it was approximately 0.8 days longer in the dominant hand group than in the non-dominant hand group (duration: 9.79 days in the dominant-side group vs. 9.12 days in the non-dominant side group, P = 0.196) (Table 2).
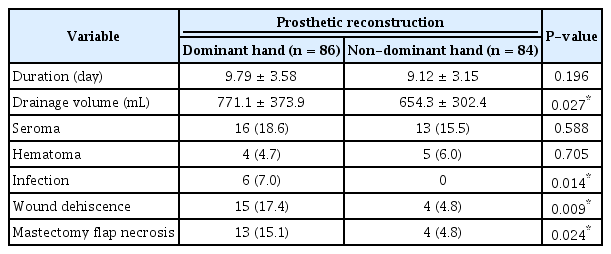
Duration and total volume of drainage and complications of the dominant and non-dominant hand sides in prosthetic breast reconstructions
The dominant hand group exhibited a high incidence of complications, including infection, wound dehiscence, and mastectomy flap necrosis. However, there was no significant difference in the incidence of seroma and hematoma within this group. In the dominant group, there were six cases (7.0%) of infection, while no cases were reported in the non-dominant hand group (P = 0.014). Wound dehiscence was observed in 15 cases (17.4%) in the dominant hand group, compared to four cases (4.8%) in the non-dominant hand group (P = 0.009). Similarly, mastectomy flap necrosis was found in 13 cases (15.1%) in the dominant hand group and four cases (4.8%) in the non-dominant hand group (P = 0.024). In the TRAM reconstruction group, we also analyzed drainage volume, drain duration, and complications. Unlike the prosthetic group, the results were not influenced by the use of the dominant hand (Table 3).
DISCUSSION
The impetus for this study was based on observations that breast reconstruction conducted on the side of the patient’s dominant hand was linked with a higher rate of seroma formation and complications. Factors such as axillary dissection, chest wall movement, lymphatic disruption, and inflammation have been associated with seroma formation in mastectomies without reconstruction [8-10]. The presence of a detectable seroma significantly elevates the risk of complications, including infection and explantation, in comparison to cases without a seroma [7]. Therefore, identifying the cause of seroma formation is crucial for preventing development and related complications.
Conflicting evidence has been derived from the analysis of seroma fluid in patients who have undergone mastectomy. Laboratory studies have suggested that seroma is similar to lymph due to its low cell content, low protein levels, and lack of fibrinogen [12-15]. Conversely, others have proposed that seroma is an inflammatory exudate, corresponding to the inflammatory phase of wound repair [16-20]. Further research is required to gain a better understanding of the nature of seroma and its formation in order to prevent associated complications.
Inflammation is not only linked to seroma formation but also to a range of other complications. These include infection, delayed wound healing, dehiscence, extended hospital stays, implant failure, the necessity for additional surgery, delayed adjuvant therapy, and a subsequent rise in overall costs [3]. There are several factors associated with inflammation following a mastectomy and prosthetic breast reconstruction, including a state of tissue hypoxia, which can worsen impaired wound healing, a foreign body reaction triggered by the tissue expander and ADM, poor ADM integration (which can be related to obesity, extended drain duration, infection, and implant loss), and surgical trauma [11,21,22]. The impact of a patient’s dominant hand use on inflammation and complications has not been documented, but it’s crucial to consider its potential influence.
Interestingly, in the group that underwent autologous TRAM, the use of the dominant hand did not influence the volume of drainage, the duration of drainage maintenance, or other complications. This was the case despite statistically significant differences in BMI and axillary node dissection between the two groups. Patients who underwent breast reconstruction with autologous TRAM were typically confined to bed for 3 to 5 days postoperatively to facilitate healing of the abdominal wound. As such, immediate postoperative immobilization likely restricted the use of the dominant hand, which could have contributed to the observed outcomes.
Prior research has indicated that early shoulder exercise can influence the development of seroma formation following a mastectomy. Postponing shoulder exercise after surgery has been shown to decrease the occurrence of seroma formation and enhance wound healing [23]. It has been reported that early and vigorous postoperative arm exercise can lead to increased fluid accumulation, while delaying shoulder movement can decrease the incidence of seroma [24]. A recent systematic review of 12 randomized controlled trials corroborates the effectiveness of a delayed arm exercise program in reducing seroma incidence [25]. When compared to immediate postoperative arm movement, a delayed exercise program can effectively decrease seroma formation without leading to long-term shoulder dysfunction.
In this study, patients were instructed to refrain from using their dominant arm and to diligently observe the drain system to minimize the impact of dominant hand usage. Furthermore, the use of supportive garments like surgical bras, arm slings, and elastic bandages could potentially decrease the use of the dominant hand and restrict chest wall movements, thereby providing extra assistance in averting complications.
This study had several limitations. Its retrospective design precluded the analysis of all risk factors for complications following breast reconstruction. However, similar risk factors were found between the dominant hand and non-dominant hand groups in patients who underwent prosthetic breast reconstruction. The skill level of breast oncologic surgeons can also influence the survival rate of the mastectomy flap. Although the surgeons involved in this study had varying levels of experience, the effect of this variable could not be statistically controlled. In this study, two relatively less-experienced breast oncologic surgeons performed 26 cases, with four cases of mastectomy flap necrosis being reviewed (2 cases in the dominant hand group, 2 cases in the non-dominant hand group). Conversely, the remaining two surgeons performed 133 mastectomies, with 13 cases of mastectomy flap necrosis (11 cases in the dominant hand group, 2 cases in the non-dominant hand group). Given the low incidence of mastectomy flap necrosis among less-experienced surgeons, it could be inferred that the surgeon’s experience does not significantly impact the outcome. Furthermore, since all the breast oncologic and reconstruction surgeons were right-handed, the surgeon’s dominant hand did not influence the study results. The study groups, particularly the autologous TRAM group, were small, leading to statistically non-significant results within this subgroup.
In conclusion, the occurrence of complications such as infection, wound dehiscence, and mastectomy flap necrosis following prosthetic reconstruction is higher in the breast on the side of the patient’s dominant hand. We hypothesize that the inevitable increased movement of the shoulder and pectoralis due to the use of the patient’s dominant hand may lead to a larger amount of discharge in the early postoperative period and subsequent inflammation. As such, we suggest considering the use of the dominant hand as a risk factor for complications in prosthetic breast reconstruction. Despite the total drainage volume being larger in the group with the dominant hand, the difference in the incidence of seroma was not statistically significant. This indicates that the closed negative drain system was effective and managed appropriately [10].
On the basis of the study findings, we recommend meticulous management of drains, compression, and early immobilization during the immediate postoperative period, especially for the breast on the dominant hand side. For patients who have undergone implant-based breast reconstruction on the dominant hand side, limiting shoulder and hand movement can be beneficial in preventing complications such as wound dehiscence, mastectomy flap necrosis, and infection.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Bundang CHA Medical Center (IRB No. 2022-11-051) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this design is a retrospective study.