Relapsed Bilateral Gigantomastia Caused by Pseudoangiomatous Stromal Hyperplasia after Reduction Mammoplasty
Article information
Abstract
Gigantomastia is an abnormal proliferation of breasts by excessive mammary tissue. Pseudoangiomatous stromal hyperplasia (PASH) is a rare benign disease due to nonspecialized fibrous mammary stroma. The incidence of gigantomastia caused by bilateral diffuse PASH is extremely rare. The authors experienced a unique case of recurrent PASH-caused gigantomastia after reduction mammoplasty. Recurrent PASH-caused gigantomastia has never been reported in the literature so far. A 33-year-old woman who suffered of gigantomastia underwent bilateral reduction mammoplasty 4 years ago. Recurrence occurred, and she visited our department. Both breasts were dense without palpable mass. Mammography revealed extremely dense breasts with a bilateral complex glandular pattern. Mastectomy with Wise-pattern incision line was performed. Nipple was reconstructed at the same time using the triangular skin flaps. Pathologic examination revealed numerous slit-like stromal clefts lined by endothelial-like spindle cells were present in well demarcated nodules and diffuse hyperplastic stromas. The finding was consistent with PASH. Reconstruction of aesthetic breast was impossible due to thinned remaining skin and subcutaneous fat tissues. Nevertheless, patient was satisfied, for her anxiety about relapse and discomfort was gone.
INTRODUCTION
Gigantomastia is characterized by excessive mammary tissue growth [1]. Patients with gigantomastia experience physical and psychological discomfort, such as pain, skin ulceration, loss of nipple sensation, and social stigma. It mainly occurs during puberty or pregnancy.
Pseudoangiomatous stromal hyperplasia (PASH) is a rare benign proliferation of nonspecialized fibrous mammary stroma [2]. It is usually asymptomatic and found incidentally on breast biopsies of premenopausal women. Although rare, PASH sometimes develops into unilateral breast nodules or masses [2,3]. However, bilateral diffuse PASH that causes rapid breast enlargement without forming discrete nodular masses is extremely rare, and has hardly been reported in the literature.
The etiology of PASH has not been well established. It is known to express progesterone and estrogen receptors, indicating that it is hormone-sensitive [3]. Pathologically, it is characterized by anastomosing slit-like pseudovascular spaces lined by bland spindle-shaped cells [4,5].
Gigantomastia due to PASH is very rare. Fewer than 10 cases have been reported in the literature, and no recurrent cases have previously been described. In this article, we report a case of recurrent gigantomastia in a patient who had received primary reduction mammoplasty at another hospital, resulting in D-cup breasts. Recurrence occurred as rapidly progressing breast enlargement. In the secondary operation performed at our center, the entire stromal tissue was totally resected. After resection of the stromal tissue, the remaining thinned skin and soft tissues were not abundant enough to reconstruct the breasts with sufficient volume. However, the patient was satisfied because her anxiety about relapse was relieved and her discomfort was resolved.
CASE REPORT
A 33-year-old woman presented with relapsed bilateral gigantomastia without pain (Fig. 1). She had systemic lupus erythematosus as an underlying disease, and had given birth 9 years ago. Her breasts were originally B-cup, but they had started to grow excessively 7 years previously. She underwent bilateral breast reduction surgery 4 years beforehand at another hospital, and the surgical procedure was presumed to be a vertical reduction mammoplasty with no pathological study. Immediately after surgery, her breasts were reduced to D-cup size, but rapid growth soon occurred, with her breasts reaching I-cup size at 4 years postoperatively. She was subsequently referred to our breast assessment center for recurrent gigantomastia.
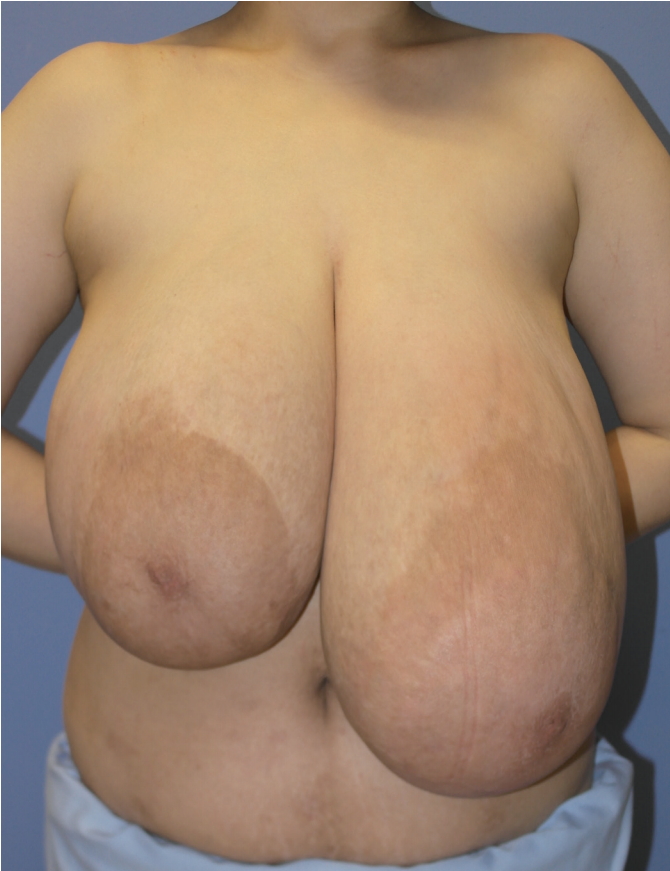
Preoperative findings. Both breasts were markedly enlarged, with no discrete, palpable masses or lymphadenopathy.
In preoperative measurements, the distance from the mid-clavicle to the nipple was 38 cm on the right side and 46 cm on the left side. Upon physical examination, both breasts were markedly enlarged with no discrete, palpable mass or lymphadenopathy. Mammography showed extremely dense breasts with a bilateral complex glandular pattern limiting the sensitivity of the study, which revealed no focal suspicious areas (Fig. 2).
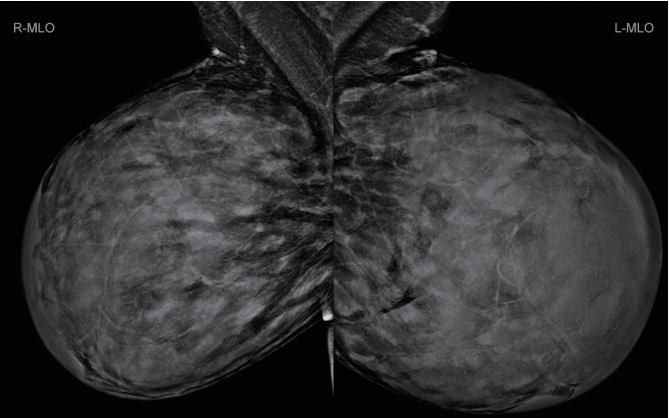
Preoperative mammography. Mammography showed extremely dense breasts with a bilateral complex glandular pattern limiting the sensitivity of the study, which revealed no focal suspicious areas.
Due to continuous enlargement of the breasts, the patient underwent bilateral mastectomy on January 2018. A Wise-pattern incision line was designed from the new nipple sites to the inframammary folds, and mastectomy with an inverted-T scar was planned. In the newly designed breasts, the length from the nipple to the mid-clavicle was 19 cm, with a nipple-to-midline distance of 9.5 cm. The length from the new nipple to the inframammary fold was 7.5 cm on both sides (Fig. 3).
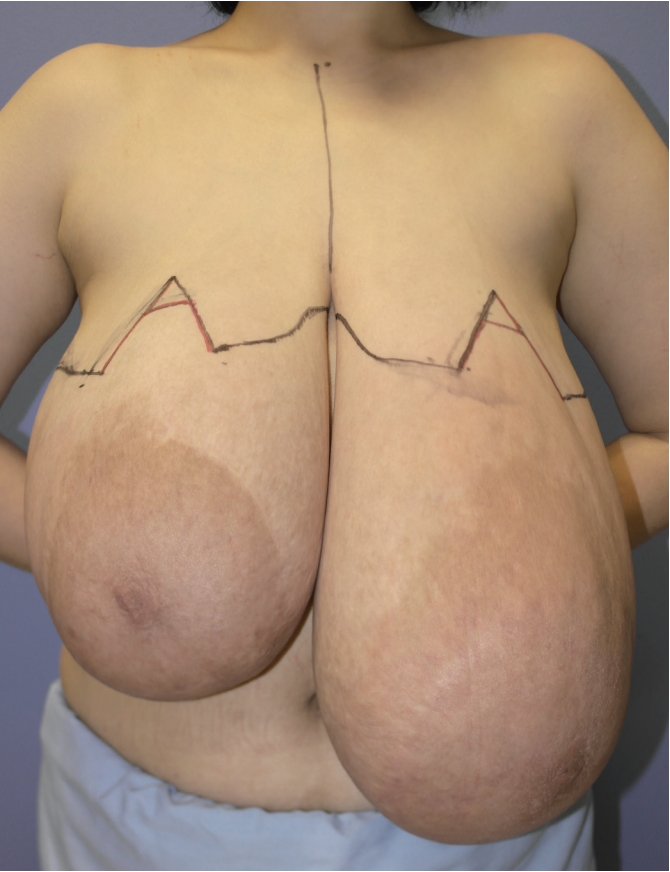
Preoperative design. A Wise-pattern incision line was designed from the new nipple sites to the inframammary folds, and mastectomy with an inverted-T scar was planned.
Under general anesthesia, an incision was performed using a #10 scalpel along the incision line, and the stromal tissue was dissected using a harmonic scalpel and resected entirely along the interface. Lobulated stromal tissues were observed in the breasts during surgery. A triangular skin flap was placed at the position of the new nipple, and nipple reconstruction was performed simultaneously (Fig. 4).
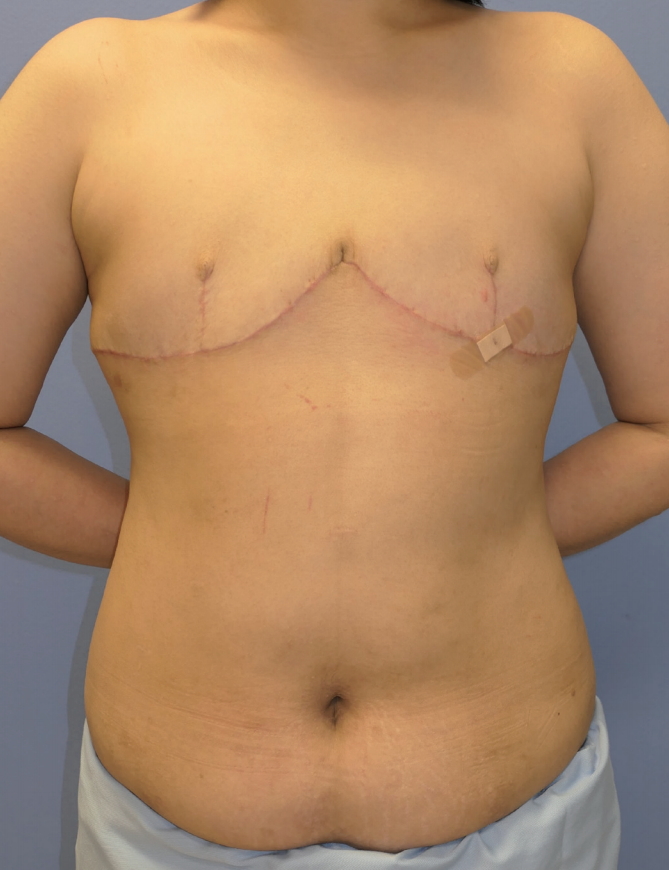
Findings at 1 month postoperatively. To avoid recurrence, the entire stromal tissue was totally resected. The remaining thinned skin and soft tissue were not abundant enough to reconstruct the breasts with sufficient volume.
The excised specimens showed tightly stretched overlying skin with diffuse nodular and edematous cut surfaces (Fig. 5). The cut surfaces revealed numerous pale-pink firm nodules of various sizes. The right excised breast tissue weighed 2,794 g and measured 21.0×15.0×13.0 cm3, with an overlying skin fragment of 20.0×13.0 cm2. No tumor was found. The left resected breast weighed 4,520 g and measured 23.0×22.0×20.0 cm3, with an overlying skin fragment of 23.0×20.0 cm2.

Postoperative specimen. The excised specimens showed tightly stretched overlying skin with diffuse nodular and edematous cut surfaces. The cut surfaces revealed numerous pale-pink firm nodules of various sizes.
On microscopic examination, numerous slit-like stromal clefts were present in grossly well-demarcated nodules and diffuse hyperplastic areas of stroma (Fig. 6). The slit-like spaces were lined by endothelial-like spindle cells with bland nuclei. The spaces of slit-like clefts were empty without red blood cells, in contrast with the vascular spaces. There was no in situ carcinoma or invasive malignancy.
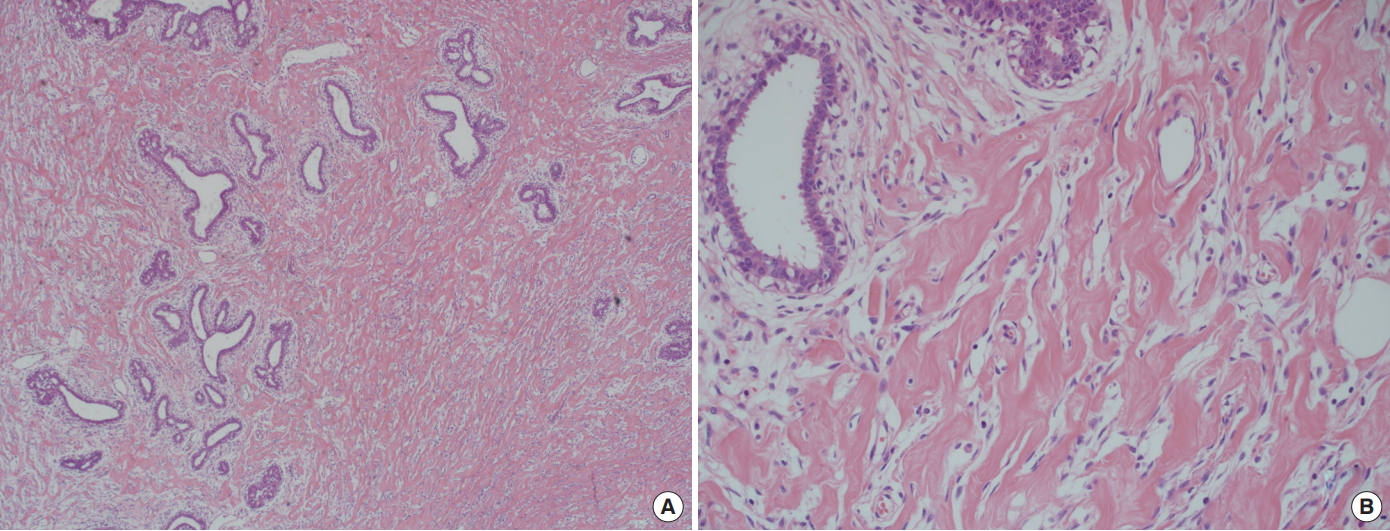
Microdescription of pathology. On microscopic examination, numerous slit-like stromal clefts were present in grossly well-demarcated nodules and diffuse hyperplastic areas of stroma. These pseudovascular spaces were not true vascular structures, as they did not contain erythrocytes. (A) Hematoxylin and eosin stain (H&E), ×40. Prominent slit-like stromal clefts are present in the hyperplastic stroma. (B) H&E, ×200. The clefts are lined by bland stromal myofibroblasts with small bland nuclei.
DISCUSSION
Gigantomastia refers to excessively large breasts due to the overgrowth of mammary tissue [1]. Patients with gigantomastia suffer from physical problems, such as mastalgia; ulceration; infection; postural problems; lower back pain; chronic traction injury of the fourth, fifth, and sixth intercostal nerves with resultant loss of nipple sensation; and psychological problems such as social stigma.
The cause of gigantomastia is not known. In a previous study of 115 patients with gigantomastia, 49.6% were found to have juvenile gigantomastia, while 35.6% were found to have pregnancy-induced gigantomastia. Other cases were idiopathic and drug-induced [6]. The results of that study, which indicated puberty and pregnancy to be the major causes of gigantomastia, imply that gigantomastia might be related to endogenous hormonal stimulation. Although hormonal stimulation is considered to cause stromal and ductal hyperplasia with dilatation, it can also cause glandular hyperplasia, hyperplasia of the stromal elements, and fibroadenoma.
PASH is a rare benign mesenchymal proliferative lesion of the breast that occurs mostly in premenopausal women [3,7]. It is found incidentally in approximately 23% of breast biopsies [8], but it is rare for it to manifest clinically. The nodular type of PASH that forms a benign mass is solitary or multifocal in a unilateral breast, with sizes ranging from 0.8 to 11 cm [3]. It mimics fibroadenoma and is rare, as fewer than 200 cases have been reported. Bilateral diffuse PASH causing rapid breast enlargement is even rarer, with fewer than 20 cases reported, including no pregnant women.
The hypothesis that PASH is related to elevated hormone levels is controversial. However, it seems possible since PASH mostly occurs in patients with hormonally active conditions. A case of hyperestrogenemia has been reported in a patient with PASH-caused gigantomastia and systemic lupus erythematosus [9]. This hypothesis is also supported by a study that proposed that PASH frequently expresses progesterone and estrogen receptors, with 95% of stain tests being positive [3]. It has also been reported that genetic disorders such as neurofibromatosis type 1 or immunosuppression (e.g., cyclosporine treatment or human immunodeficiency virus) could cause PASH [10].
Histologically, PASH is observed as a complex network of slit-like spaces lined by discontinuous endothelial-like flat spindle cells without mitosis or nuclear atypia [4,5], separated by dense collagenous and fibrous areas of stroma. However, these pseudovascular spaces are not true vascular structures, as they contain no erythrocytes. The immunophenotypic profiles of stromal cells in PASH are all positive for D34, B-cell lymphoma 2, CD99, and vimentin [11]. It is important to distinguish PASH histologically from fibroadenoma or low-grade angiosarcoma Additional differential diagnoses include a variety of lesions, such as fibrocystic changes, phyllodes tumors, ductal carcinoma in situ, and invasive carcinoma [8].
Imaging studies of PASH usually show non-specific findings [2]. Mammography does not reveal any abnormalities in most cases, but a solitary, non-calcified mass may be present. On imaging studies, PASH should be differentiated from fibroadenoma, especially in young patients, and phyllodes tumors in older women [3,7].
In terms of prognosis, PASH has been reported to exhibit slow growth without significant changes [7]. However, there has been a report of a mass with a diameter of 1.0 cm growing rapidly to 5.0 cm in a month [12]. Although PASH is generally not considered to be a malignant or premalignant lesion, malignant changes such as low-grade myofibroblastic sarcoma [4], ductal carcinoma in situ [12], and invasive carcinoma [7] have been reported.
For the treatment of PASH, the current recommendation is to excise lesions that are larger than 2 cm in diameter. Close observation is recommended in smaller lesions. Axillary dissection is not recommended since lymph node invasion is rare. The effect of tamoxifen is controversial because it can cause both tumor regression [13] and tumor growth [14].
The patient in our case underwent primary reduction mammoplasty at another hospital and was referred to our breast center for recurrent gigantomastia. Among patients with an enlarged breast due to PASH, gigantomastia is very rare, and there has been absolutely no report of recurrent gigantomastia due to PASH until now. The authors consider that the recurrence of gigantomastia was caused by PASH of the remaining mesenchymal tissue after the primary operation. Thus, in our second operation, the lobulated stromal tissue was completely excised in an effort to prevent any recurrence.
After complete resection of the stromal tissues, the remaining thinned skin and soft tissues were not abundant enough to reconstruct the breasts with sufficient volume, making the final appearance of the breasts too small to be aesthetically ideal. However, the patient was satisfied, since her anxiety about relapse was eliminated and her discomfort due to enlarged breasts was resolved. A previous report described aesthetically pleasing results after Wise-pattern mastectomy of PASH-caused gigantomastia followed by implant insertion [15]. Implant insertion could be considered in our patient as well, if the patient eventually desires more aesthetically pleasing breasts.
Acknowledgements
This article was presented at the 8th Research and Reconstruction Forum on April 19–20, 2018 as a poster.
Notes
No potential conflict of interest relevant to this article was reported.
PATIENT CONSENT
Patient provided written consent for the use of her images.