Complications of polyacrylamide hydrogel injection for breast augmentation: A case report and literature review
Article information
Abstract
Polyacrylamide hydrogel (PAAG) was developed in the 1980s as an injectable filler for breast augmentation and tissue contour improvement, but its potential risk for oncogenesis and the frequent occurrence of chronic complications after injections led to the prohibition of its further use as an injectable material. Although breast augmentation with PAAG injections was mostly performed in China and Eastern Europe, the migration of patients and long-term complications of the procedure made it a global concern. Herein, we describe the case of a 49-year-old woman who immigrated to Korea after undergoing breast augmentation via PAAG injection in China, and complained of persistent mastodynia and retraction of both breasts. Surgical treatment was undertaken, along with removal of the PAAG and total capsulectomy of the fibrous capsule containing the gel through an inframammary fold incision. We share our experience of diagnosing and treating this case, and present a literature review.
INTRODUCTION
Polyacrylamide hydrogel (PAAG) injections were widely used in China and Eastern Europe in soft tissue augmentation procedures for esthetic purposes until numerous reports of complications were published, and consequently China banned the medical use of this product in 2006. The PAAG used for breast augmentation procedures via injection was sold under various product names, including Amazing Gel, Aquamid, Interfall, Formacryl, Bioformacryl, Bio-alcamid, and Argiform [1]. Although debates on the potential oncogenic effect of PAAG have not reached a definitive conclusion, complications of PAAG injections have affected numerous patients, causing esthetic dissatisfaction, mastodynia, inflammation, and infection [2]. With increasing migration and ethnic diversity, plastic surgeons are becoming more aware of PAAG, but no concrete consensus has been reached in Korea regarding the proper management of patients who have undergone breast augmentation via PAAG injection.
Herein, we report a case of a patient who received PAAG injection for a breast augmentation procedure in China before immigrating to Korea. We report the treatment process, including the preoperative diagnosis with a magnetic resonance imaging (MRI) scan and surgical management with total capsulectomy of the fibrous capsule surrounding the PAAG, along with an overall review of the related literature on breast augmentation via PAAG injection.
CASE REPORT
A 49-year-old woman presented to our clinic who had undergone bilateral breast augmentation with injectable material (Amazing Gel) in 2000. She underwent the procedure in Yanji, China, before immigrating to Korea. The patient could not remember the amount of the injected material. Her main complaints were persistent mastodynia and retraction of both breasts for a year prior to her presentation at our clinic. Migration of the gel over time and consequent breast asymmetry and change of the position of the nipples were observed by the patient (Fig. 1). A clinical examination revealed hardening of both breasts, limited movement, and fixation to the underlying structure. Otherwise, there were no signs of infection, nipple drainage, or inflammation.
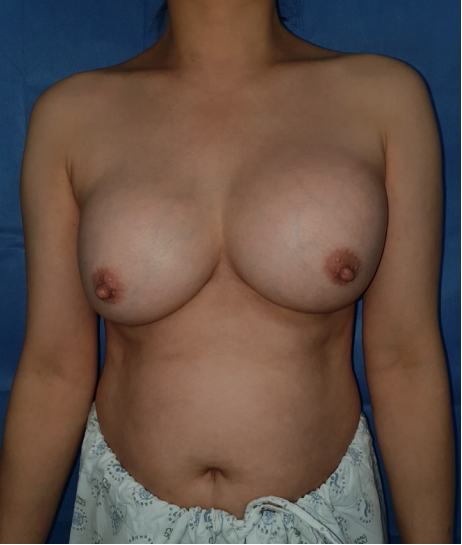
Preoperative photo of the patient. Asymmetrical size and shape of the breast and nipple positions were identified.
Preoperative mammography revealed calcified nodular lesions on both breasts. Breast ultrasonography indicated the presence of multiple hypoechoic masses along the lining of the injected foreign body material in both breasts. Preoperative MRI revealed that the fluid-filled foreign bodies had aggregated to form a unified material similar to a silicon implant. The foreign body material showed a low signal on T1-weighted images and a high signal on T2-weighted images. Axial silicone-suppression MRI for visualizing silicone implants yielded high signal intensity, confirming that the injected material was not composed of silicone. Nodular enhancing lesions along the capsule lining of both breasts were identified in the breast MRI scan (Fig. 2).
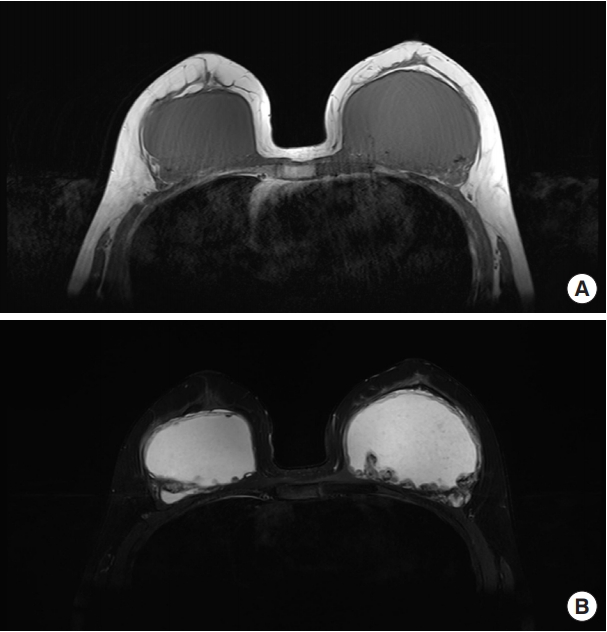
Preoperative breast magnetic resonance imaging of the patient. (A) T1-weighted turbo spin echo (TSE) transverse scan image showing a low signal of the injected polyacrylamide hydrogel (PAAG) on both breasts. (B) T2-weighted TSE transverse scan image showing a high signal of the injected PAAG on both breasts.
Surgery for foreign body removal was planned. It was performed with the patient lying in a supine position. An incision was made along the inframammary fold to access the subglandular space to remove the foreign body. Dissection was performed to reach the outer surface of the fibrous capsule, which contained the PAAG (Fig. 3A). The layer between the outer surface and glandular tissue was dissected to isolate the capsule cautiously to avoid spilling the gel. The capsule was firmly attached to the pectoralis major muscle on the left breast, suggesting that there was diffuse infiltration through it. The pectoralis major muscle fibers were dissected to reveal the injected cavity, and as much of the PAAG as possible was removed. The intraoperative findings showed that the PAAG resembled a purée-like yellowish material (Fig. 3B). Total capsulectomy, with the removal of all areas containing the PAAG, was performed on both breasts (Fig. 3C and D). Curettage was repeatedly performed to remove areas of residual gel that formed nodules. All excised capsule tissue was sent for a pathological examination. Extensive lavage was performed with normal saline mixed with antibiotics. A drain was placed on each breast and the wound was closed in layers.
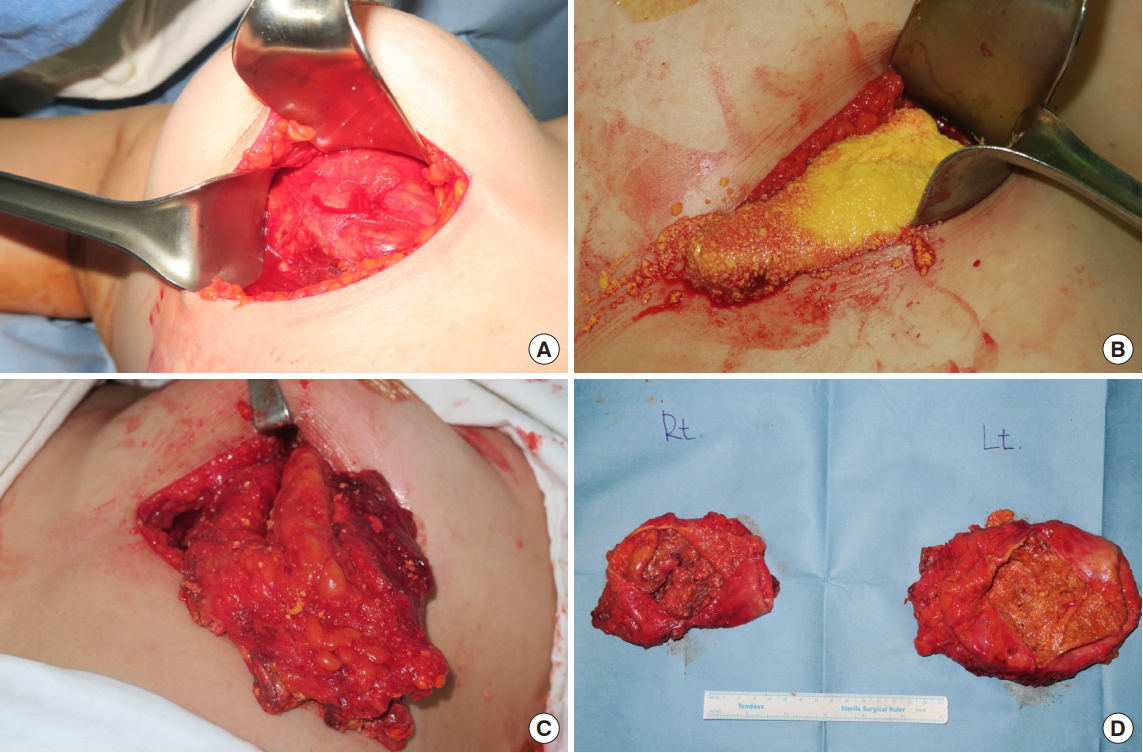
Intraoperative findings. (A) Fibrous capsule formation surrounding the polyacrylamide hydrogel (PAAG) injected into the right breast. (B) Purée-like yellowish PAAG flowed out of the torn PAAG capsule of the left breast. (C) Total capsulectomy of the PAAG-containing capsule in the left breast. (D) Bilateral PAAG-containing capsules after total capsulectomy of both breasts.
The patient was discharged on postoperative day 3 after the drain was removed without any complications. At a 1-year follow-up at our outpatient clinic, the patient’s operative site remained stable without recurrence of mastodynia or retraction of the breasts. The patient was satisfied with the loss of foreign body sensation and restoration of the breasts’ softness, and did not wish to undergo an reconstructive procedure (Fig. 4). In the pathological examination, the excised tissue was stained with hematoxylin and eosin, showing multiple giant cells and calcification, indicating the presence of a foreign body reaction with the polyacrylamide gel, which was stained as a purplish amorphous material (Fig. 5A). Fibrous connective tissue hyperplasia was identified surrounding the PAAG aggregation (Fig. 5B).

Postoperative photos of the patient 1 month after surgery. Mastodynia and retraction of breasts resolved after removal of the polyacrylamide hydrogel.
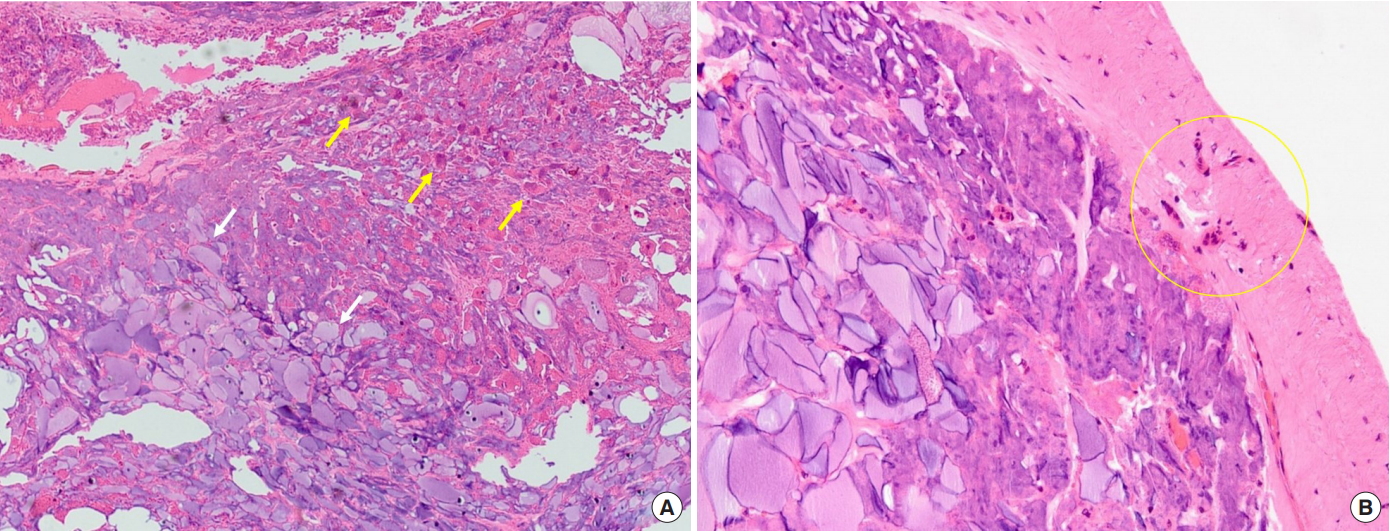
Histopathological examination of the breast parenchyma injected with polyacrylamide hydrogel (PAAG). (A) Multiple giant cells (yellow arrows) and calcification surrounding the amorphous basophilic PAAG (white arrows) showed a foreign body reaction (H&E, ×40). (B) Fibrous capsule formation around the PAAG was identified (yellow circle) (H&E, ×100).
DISCUSSION
We present the case of a 49-year-old woman who immigrated to Korea after undergoing breast augmentation through a PAAG injection in China and complained of persistent mastodynia and retraction of the breasts. Surgical treatment was undertaken, along with removal of PAAG and total capsulectomy of the fibrous capsule containing the gel through an inframammary fold incision.
PAAG consists of 2.5%–5% cross-linked polyacrylamide and 95%–97.5% non-pyogenic water, and was initially manufactured in Ukraine [3]. After the development of PAAG, breast augmentation via PAAG injection became popular in Eastern Europe, Russia, Iran, and China, as it was cheap and feasible, requiring neither general anesthesia nor dexterous surgical skills [4,5]. More than 300,000 women underwent the procedure from 1997 to 2006 in China with the approval of the Chinese State Food and Drug Administration [6].
Although the association between augmentation procedures with PAAG and cancer has not been conclusively elucidated, there have been reports of breast cancer incidence after mammoplasty post–PAAG injection [7,8]. Acrylamide monomer, which is a residue after the synthesis of PAAG (present in the product at a proportion of 0.1%–1%) and can be potentially generated from degradation in human body, is considered to be a potential carcinogen due to its neurotoxicity and teratogenicity [8]. In addition, PAAG has been reported to disturb the physical parameters of human fibroblasts, altering their size and the granularity and increasing c-myc mRNA expression, suggesting its potential carcinogenicity [2].
Other complications reported in the literature include induration, lumps, infection, persistent mastodynia, poor cosmetic results, gel migration, hematoma, and the potential for a delayed diagnosis of breast cancer [2]. With regard to breastfeeding, the gel has been known to cause acute inflammation and the formation of galactoceles, which are suspected to occur secondary to fibrosis and blockage of ducts and can act as a medium for bacterial proliferation [9].
Single-stage reconstruction can be done with the removal of PAAG if the patient strongly prefers, but the presence of acute inflammation and infection is a major factor that may make it necessary to perform a two-stage reconstruction [4]. Preoperative MRI is the gold standard for determining the presence of inflammation. When inflammatory signs are absent, the MRI signal of polyacrylamide gel is hypointense on the T1 sequence and hyperintense on the T2 sequence, with a thin hypointense T2 rim. If there is an inflammatory change, the intensity of the T1 sequence increases and that of the T2 sequence decreases, resulting in an intermediate signal, together with irregular and thickened rim enhancement [10].
Successful treatment of complications of PAAG augmentation lies in the maximal removal of PAAG. Prior to surgery, it should be clearly explained to the patient that absolute surgical removal of PAAG is impossible. The inframammary fold approach or periareolar approach can be used. While a periareolar incision provides access to PAAG that has migrated into the infraclavicular region, an inframammary fold incision is more beneficial for the removal of hydrogel within the breast region. The surgical findings often include a capsule and fibrous septum between lesions. When the fibrous capsule infiltrates the pectoralis major muscle, it is advisable to remove en bloc the infiltrated fascia and muscle. The blunt aspiration technique using negative suction, although it is widely performed, is an ineffective method for the maximal removal of infiltrated PAAG [11].
Previous histopathological findings of PAAG capsules revealed fibrous connective tissue hyperplasia, degeneration of breast tissue, formation of fibrous tissue, and fiber contraction, leading to displaced papillae and mastodynia [12]. Another report revealed macrophages and giant cells surrounding the PAAG, suggesting a foreign body reaction, similar to our finding [13].
The impact of PAAG on the Asian population has been underestimated compared to paraffin and liquefied silicone, which posed significant issues in terms of complications for plastic surgeons in the United States and Europe. Compared to Western societies, implant-based breast augmentation procedures were introduced to the public and become popular in Asia a few decades later, due to recent technical developments and patients’ increasing ability to pay for these procedures. Given the low levels of knowledge and information regarding PAAG, its inexpensive cost and the simplicity of the procedure led people to opt for this hazardous procedure. As immigration from Asia to the rest of the world becomes a trend and globalization rapidly progresses, meaning that plastic surgeons practicing anywhere in the world can encounter patients with a history of breast augmentation via PAAG injection, it is appropriate to provide this reminder to the global community of patients and physicians of the complications and surgical handling of PAAG.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of SMGSNU Boramae Medical Center (IRB No. 10-2019-15) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of her images.
