Histologic determination of apocrine gland depth in patients with axillary osmidrosis
Article information
Abstract
Background
Subdermal shaving is a surgical procedure for the treatment of axillary osmidrosis. In this procedure, a direct axillary incision is made, and the apocrine glands are removed. Insufficient subcutaneous tissue removal during subdermal shaving can lead to recurrence due to the presence of remaining apocrine glands, while excessive removal can injure the subdermal plexus and cause skin necrosis. We measured the depth of the apocrine glands from the basement membrane of the epidermis to develop a quantitative method of determining the thickness of the skin flap to be removed.
Methods
A chart review of patients who underwent subcutaneous shaving to treat osmidrosis between 2012 and 2019 was performed. Axillary subcutaneous tissues were harvested from five randomly chosen patients with osmidrosis before and after surgery. The apocrine gland depth was then measured via immunofluorescence (IF) staining of the collected tissues. A questionnaire was administered to 10 of the patients to investigate postoperative outcomes.
Results
Of the 47 total patients, six (12.8%) experienced recurrence, seven (14.9%) had complications, four (8.5%) had skin necrosis, two (4.3%) had hematomas, and one (2.1%) had an infection. One patient underwent reoperation due to hematoma. IF staining revealed the mean distance from the basal layer of the epidermis to the apocrine glands to be 1.4312±0.8064 mm. On the questionnaire, the mean patient rating of axillary odor was 8.6 pre-surgery and 4.4 post-surgery.
Conclusions
During subdermal shaving, the subcutaneous tissue must be preserved up to 14.312±8.064 mm from the epidermal basement membrane to remove the apocrine glands while preserving the subdermal plexus.
INTRODUCTION
Axillary osmidrosis affects 4%–8% of Asians and is more common in Caucasians [1]. Recent studies have revealed an association between wet earwax and ABCC11 genotype, suggesting that axillary osmidrosis is hereditary [1,2]. Since axillary osmidrosis is affected by sex hormones, it is less common among children and the elderly and more common among adolescents and middle-aged people [3]. Axillary sweat contains ε-3-methyl 2-hexenoic acids, which oxidize the normal flora of the apocrine glands, including Staphylococcus aureus. This creates an axillary odor [2,4]. Such odors can disrupt one’s daily life and social activities and can cause depression or social phobia in severe cases [5].
Nonsurgical procedures such as laser treatment and botulinum toxin A injection have high recurrence rates. In a recent approach, diode laser ablation is performed in conjunction with surgery to reduce recurrence [6,7]. Subdermal shaving is a surgical procedure in which the apocrine glands are directly removed. It is reported to have lower recurrence rates than nonsurgical procedures [8].
Excessive removal of subcutaneous tissue during subdermal shaving can injure the vascular plexus and lead to complications such as skin necrosis [6,8,9]. On the other end of the spectrum, insufficient removal of subcutaneous tissue can lead to recurrence due to the presence of remaining apocrine glands. We performed biopsies of axillary tissues before and after apocrine gland removal via subdermal shaving to measure the distance of the apocrine glands from the basal layer of the epidermis. We thus provide histologic evidence that can be used to determine the depth to which shaving must be performed. Additionally, we measured recurrence and complication rates by performing a chart review.
METHODS
A retrospective chart review was conducted among patients who underwent subcutaneous shaving to treat osmidrosis at the Department of Cosmetic Surgery of Yeungnam University Hospital between January 2012 and February 2019. The goal of this review was to measure recurrence, reoperation, and complication rates. Recurrence was defined as the presence of symptoms at least 3 months after surgery. Complications included skin necrosis, hematoma, and infection [8]. Axillary subcutaneous tissues were harvested from five randomly chosen patients with osmidrosis before and after subcutaneous shaving. The five patients prospectively agreed to the harvesting and analysis of their axillary subcutaneous tissue. The tissues were subjected to immunofluorescence (IF) staining using mucin, an apocrine gland marker, to measure the distance of the apocrine glands from the basal layer of the epidermis. The tissues were examined to determine whether the apocrine glands had been adequately removed and the vascular plexus was intact after the procedure. A questionnaire was administered to 10 of the patients to investigate changes in the perceived axillary odor. The present study protocol was reviewed and approved by the Institutional Review Board of Yeungnam University College of Medicine (approval no. YUMC 2020-03-038).
Surgical technique
Two 4-cm incision lines parallel to the axillary creases were planned for each axilla. A #15 scalpel blade was used to make the incision, and blunt Metzenbaum and bipolar scissors were used to dissect the subcutaneous layer. Apocrine gland-containing subcutaneous tissues were directly excised using sharp Metzenbaum scissors or removed using a Versajet apparatus (Versajet Hydrosurgery System; Smith and Nephew, Memphis, TN, USA) (Fig. 1). After copious saline irrigation, bleeding was controlled with bipolar electrocautery. Monosyn 3-0 and Surgipro 5-0 sutures were used to suture the subcutaneous layer and the skin, respectively. Two Penrose drains were inserted into each axilla. After the application of simple dressings, the surgical site was compressed with a figure-of-eight bandage.
IF staining
The specimens were embedded in optimal cutting temperature compound. The blocks were frozen and cut into 20-μm sections. The tissue sections were blocked in phosphate-buffered saline with 5% goat serum for 1 hour. Then, these sections were incubated overnight at 4°C with anti-CD31 (rabbit; ab32456, a marker of vascular endothelial cells) and anti-mucin (mouse; ab70475, a marker of apocrine glands) primary antibodies. CD31 is expressed by the endothelial cells of the subdermal plexus, and mucin is expressed by the secretory cells of the apocrine glands. After three rounds of washing with phosphate-buffered saline, the sections were incubated for 2 hours at room temperature with Alexa Fluor 488-conjugated anti-rabbit and Alexa Fluor 594-conjugated anti-mouse IgG (immunoglobulin G) secondary antibodies. Nuclei were stained with 4´,6-diamidino-2-phenylindole dihydrochloride (DAPI). A confocal microscope (LSM 800; Carl Zeiss AG, Oberkochen, Germany) was used to visualize the fluorescence images. Three points were randomly selected from each specimen, and the distances from the basal layer of the epidermis to the apocrine glands were measured.
Patient questionnaire
Of the 47 patients who underwent surgery, 10 were randomly selected to complete a questionnaire survey. The patients rated their axillary odor on a 10-point visual analogue scale before and after surgery. On this scale, 0 points indicated no axillary odor and 10 points indicated severe odor. The t-test and the Wilcoxon signed-rank test were applied to the visual analogue scale scores to compare axillary odor before and after surgery. Statistical analysis was performed using SPSS version 24 software (IBM Corp., Armonk, NY, USA).
RESULTS
Retrospective chart review
Of the total of 47 patients, 23 were male and 24 were female. The mean age of the patients at the time of surgery was 25.9 years (range, 12–64 years). The apocrine glands were removed using Metzenbaum scissors in 25 patients and using the Versajet apparatus in 22 patients (Table 1).
Six patients (12.8%) experienced recurrence, seven (14.9%) had complications, four (8.5%) had skin necrosis, two (4.3%) had hematomas, one (2.1%) had an infection, and one patient underwent reoperation due to hematoma (Table 2).
IF staining
By staining the apocrine glands with mucin, we could histologically examine the distribution of the apocrine glands. The mean distance from the basal layer of the epidermis to the apocrine glands before surgery was measured as 1.4312±0.8064 mm (range, 0.670–2.595 mm) (Fig. 2).
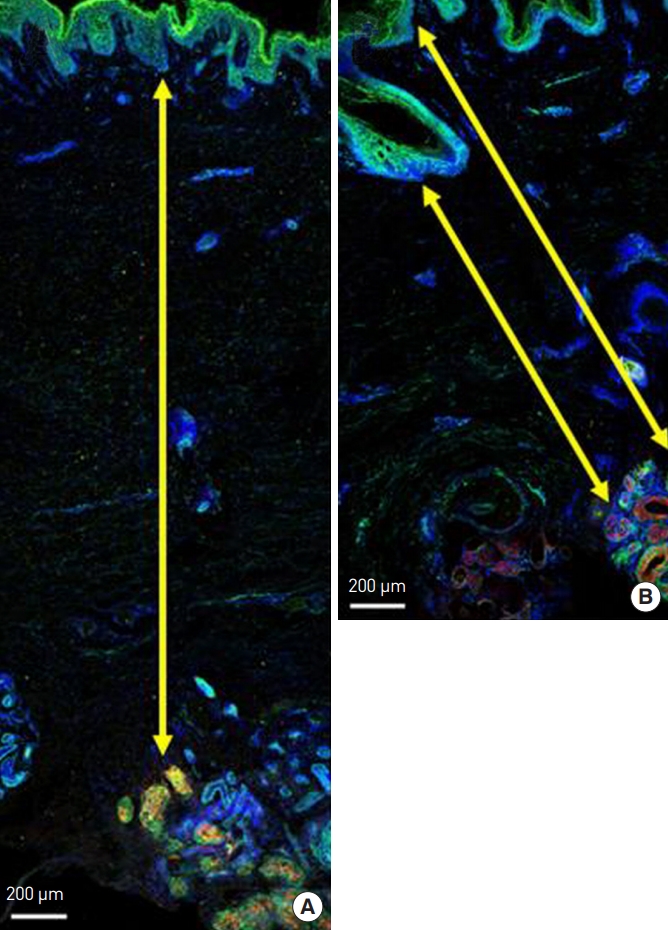
(A, B). Immunofluorescence staining of harvested axillary tissue. Secretory cells in the apocrine glands stained positive for mucin (red). Endothelial cells in the vascular plexus stained positive for CD31 (green). Nuclei were stained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) (blue). The distance from the basal layer of the epidermis to the apocrine gland is indicated by the yellow arrow.
In the postoperative immunohistological examination, the apocrine glands were observed to have been successfully removed. IF staining of harvested axillary tissues was performed before surgery to examine the apocrine glands, dyed in red, and the subdermal plexus, dyed in green (Figs. 3A, 4A). The subdermal plexus (Figs. 3B, 4B) and apocrine glands (Figs. 3C, 4C) were magnified during the examination. IF staining was performed on the harvested remnant axillary tissues after subdermal shaving (Figs. 3D, 4D). No apocrine glands were observed, indicating successful apocrine gland removal. The subdermal plexus was still intact. The preserved subdermal plexus was similarly magnified during the examination (Figs. 3E, 4E).
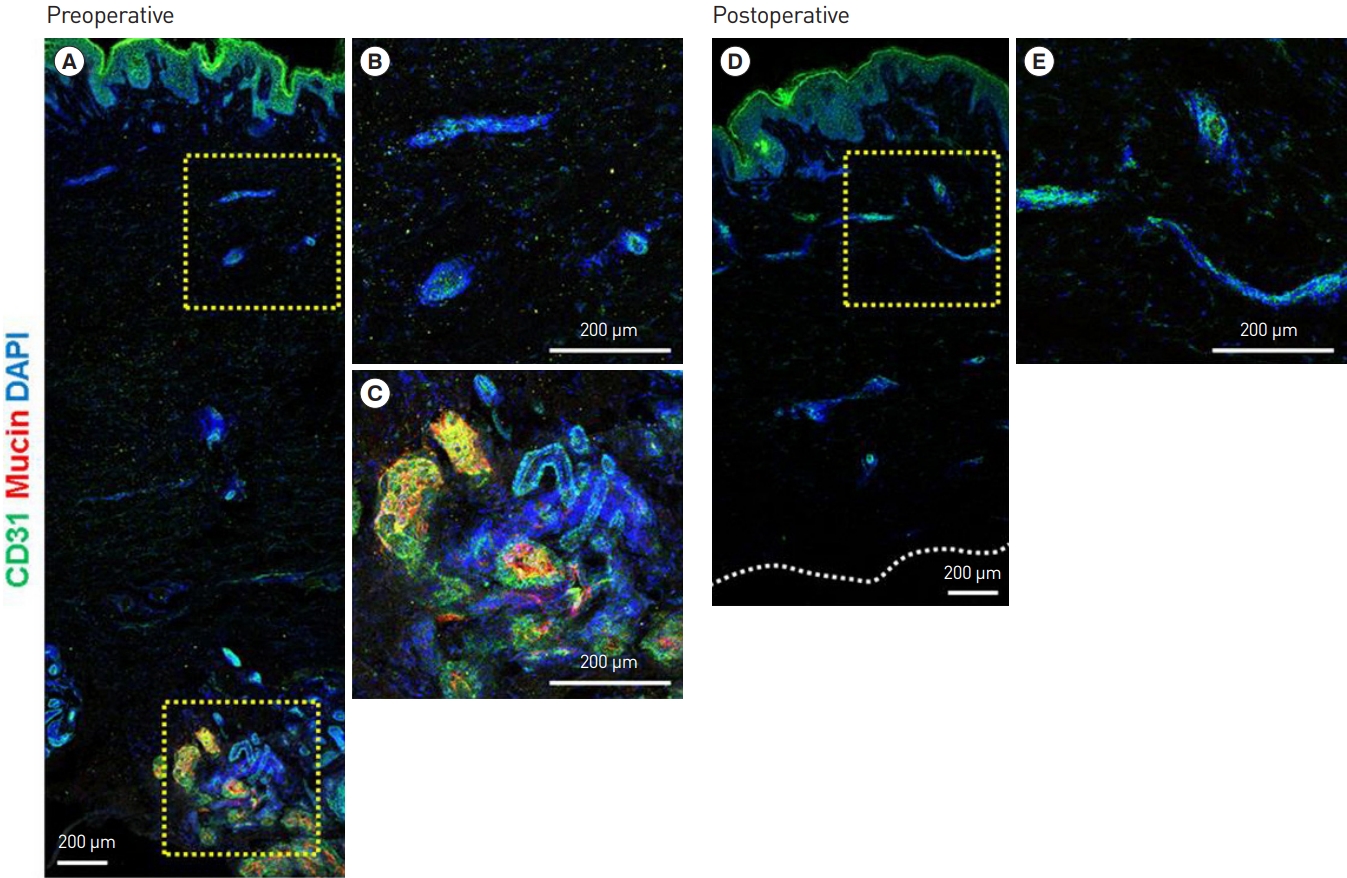
Preoperative immunohistological examination. (A) Immunofluorescence (IF) staining of human axillary tissue. (B) CD31 (green) IF staining of the subdermal plexus. (C) Mucin (red) IF staining of the apocrine glands. Postoperative immunohistological examination. (D) IF staining of human axillary tissue after subdermal shaving. (E) CD31 (green) IF staining of the preserved subdermal plexus after surgery. The apocrine glands (via mucin staining) were observed before but not after surgery, while the vascular plexus (via CD31 staining) was observed both before and after surgery (the yellow box to the right side of each figure represents a magnified region).

Preoperative immunohistological examination. (A) Immunofluorescence (IF) staining of human axillary tissue. (B) CD31 (green) IF staining of the subdermal plexus. (C) Mucin (red) IF staining of the apocrine glands. Postoperative immunohistological examination. (D) IF staining of human axillary tissue after subdermal shaving. (E) CD31 (green) IF staining of the preserved subdermal plexus after surgery. The apocrine glands (via mucin staining) were observed before surgery, but not after surgery, while the vascular plexus (via CD31 staining) was observed both before and after surgery (the yellow box to the right side of each figure represents a magnified region).
Patient questionnaire
The mean patient ratings of axillary odor were 8.6±1.0 before surgery and 4.4±1.2 after surgery. The mean decrease in the patient rating of axillary odor was 4.2±1.3, constituting a significant reduction. No patients responded that their axillary odor grew stronger after surgery. A P-value of 0.002 was determined via the Wilcoxon signed-rank test (Table 3).
DISCUSSION
Surgery is the most common method of treatment for osmidrosis. Subdermal shaving, proposed by Inaba et al. [10] in 1978, is the most well-known surgical treatment for osmidrosis. The procedure involves making an incision on the axillary skin and selectively removing subcutaneous tissue. Subdermal shaving has high rates of complications, including hematoma, skin necrosis, scar contracture, and infection. In 1978, Jemec and Holm Hansen [11] reported that subdermal shaving had a complication rate as high as 36%. Nonsurgical techniques have been developed to overcome this drawback of subdermal shaving [7-9].
Nonsurgical procedures such as liposuction, carbon dioxide laser vaporization, and botulinum toxin A injection have been proposed [8,10,11]. These procedures have lower complication rates than surgical methods but fail to effectively remove the apocrine glands. They are not effective treatments for osmidrosis due to their high recurrence and low patient satisfaction rates.
We believed that it was necessary to sufficiently remove apocrine glands in the subcutaneous tissue while preserving the subdermal plexus to prevent complications and recurrence after osmidrosis treatment. In a meta-analysis of the depth of apocrine glands, most studies were found to have reported that the apocrine glands are located at the subcutaneous tissue level near the dermis [12]. However, no study has measured the exact distance of the apocrine glands from the epidermis. In 2006, Lawrence and Lonsdale Eccles [13] reported that sweat gland lobules started at a mean depth of 2.4 mm of the 5.9-mm skin thickness in Caucasians. The researchers randomly selected five of 15 axillae and obtained those measurements through hematoxylin and eosin staining. Lawrence et al. measured the distance of sweat glands from the skin surface and explained that apocrine glands cannot be distinguished from apoeccrine glands under light microscopy. Our study was the first to measure the actual depth of the apocrine glands in axillary tissues through IF staining while distinguishing them from the apoeccrine glands.
We treated 47 patients with osmidrosis, one of whom required revision. The patient requiring revision was a 24-year-old man who underwent subdermal shaving on both axillae. The revision was performed due to a hematoma that formed on the right axilla 1 day after the shaving procedure. Skin necrosis developed following the revision, and the axilla healed by secondary intention. The remaining skin was treated without complications such as necrosis, hematoma, or infection requiring revision.
This study had a few limitations. First, six (12.8%) of the 47 patients who underwent subdermal shaving for osmidrosis experienced recurrence. Recurrence was subjectively assessed by the patients. Some patients were not aware of a reduction in their axillary odor or felt that their symptoms persisted even after surgery. Indices that can be used for the objective assessment of the odor of patients with osmidrosis may be useful in preoperative and postoperative assessments.
The depth of the apocrine glands varies between individuals of the same or different ethnicities. In this study, we histologically examined five cases of osmidrosis and found a high standard deviation in the depth of the apocrine glands. The depth of the glands could have been more precisely measured had a larger number of samples been examined.
Until now, surgeons have intuitively determined the depth of subcutaneous tissue to remove during subdermal shaving. Even if they know the average depth of the apocrine glands, it is still difficult to remove the subcutaneous tissue while evaluating the depth of the apocrine glands during an actual procedure. Similar to the near-infrared fluorescence imaging device used to check skin vascularity following an indocyanine green injection [14], a device that can be used to visualize the apocrine glands during subdermal shaving must be explored in future research.
Osmidrosis can be treated by removing apocrine glands using subdermal shaving. The subcutaneous tissue must be preserved up to 1.4312±0.8064 mm from the epidermal basement membrane to remove the apocrine glands while preserving the subdermal plexus, thereby preventing complications and recurrent osmidrosis.
Notes
IKK, an associate editor of Archives of Aesthetic Plastic Surgery, is the corresponding author of this article. However, he played no role whatsoever in the editorial evaluation of this article or the decision to publish it. Except for that, no potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Yeungnam University College of Medicine (IRB No. YUMC 2020-03-038) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and the use of their images.




