 |
 |
- Search
| Arch Aesthetic Plast Surg > Volume 29(1); 2023 > Article |
|
Abstract
Background
Keloid treatment is challenging because of the high likelihood of recurrence and a lack of definitive treatment combinations. The treatment of bulky and recurrent keloids is particularly difficult. We investigated the administration of extralesional cryotherapy (EL) in conjunction with intralesional (IL) triamcinolone (TA) injections as adjuvant therapy after surgical excision for the management of keloids.
Methods
Among all patients who visited our scar laser center between January 2016 and August 2017, 54 patients who underwent IL keloid excision with EL cryotherapy and IL TA injection as adjuvant therapy were included in this retrospective study. We examined sex, site, the number of cryotherapy sessions and TA injections, symptoms after surgery, and recurrence. The Vancouver Scar Scale (VSS) was used as to quantify treatment outcomes.
Results
Among 54 cases of IL keloid excision, after an average of 6.26 cryotherapy sessions and IL TA injections as combined adjuvant treatment, the lesion was controlled without recurrence in 49 cases. Relapse occurred in five patients, requiring additional treatment and reoperation. For 49 patients with photographic data, the average VSS score before and after treatment improved from 10.1 to 5.0. In 17 patients in whom symptoms recurred after surgery, all symptoms were controlled and maintained with adjuvant therapy.
Keloids originate from skin overgrowth beyond the original woundŌĆÖs boundaries [1]. Keloid treatment is challenging owing to the high likelihood of relapse and the lack of definitive treatment combinations. In general, wound healing occurs in nearly every tissue after exposure to almost any destructive stimulus; however, healing in response to fibrosis does not usually involve normal regeneration. Scars persisting on the skin after surgery or injury are not only esthetically undesirable but also cause functional deterioration, imposing a substantial clinical burden on patients and surgeons.
Several methods are used for keloid treatment, including surgical excision, silicone sheets, pressure therapy, intralesional (IL) corticosteroids, IL 5-fluorouracil, and radiation therapy. However, none of these modalities can completely eliminate keloids. Cryotherapy was first described for skin lesions by Weshahy [2] in 1993, while Zouboulis and Orfanos [3] reported the use of cryotherapy for scar treatment in 2000. It was subsequently adopted and developed for keloid treatment by Har-Shai et al. [4,5].
A keloid scar with a narrow base is the ideal target for this treatment modality, as the narrow base concentrates the cooling effect on the small soft-tissue pedicle to maximize the freezing effect [6]. Cryotherapy comprises two phases: physical and vascular. During the physical phase, rapid freezing causes direct cellular damage via sharp ice crystal formation. Moreover, differential freezing of the cellular compartments alters the osmotic pressure gradient and causes electrolyte imbalance, leading to irreversible cellular damage. The impairment of microcirculation and failure during the vascular phase lead to cell destruction via ischemic necrosis [7]. Cryotherapy results in early lesion flattening due to microvascular damage caused by tissue hypoxia and altered collagen synthesis and promotes fibroblast differentiation in keloid tissue to the normal phenotype [8]. Histochemical studies of post-cryotherapy tissue have revealed that the noduleŌĆÖs collagen fiber structure is composed of proliferating fibroblasts embedded in dense collagen bundles, and a more organized, parallel arrangement of the eosinophilic collagen bundles with thickened keloid fibers. Cryotherapy results in prominent vascular proliferation and a reduced number of mast cells [9].
Existing cryotherapy applicators that use liquid nitrogen often cause undesirable hypopigmentation or scarring, since their freezing point is below ŌĆō196 ┬░C; the CryoPenŌĆÖs freezing point is below ŌĆō79 ┬░C. The CryoPen prevents tissue destruction and epidermal damage, while potentially inducing fibroblast inactivation and facilitating ease of handling due to device miniaturization [8]. Since the CryoPen induces less pain and discomfort than conventional cryotherapy devices using an IL spray at ŌĆō196 ┬░C, local anesthesia is not required, and the psychological burden on the patient is lower [10].
IL triamcinolone (TA) injection constitutes the first-line therapy for keloids; it effectively reduces alpha-globulin deposition in keloid tissue and inhibits fibroblast growth [8]. Keloid scarring has been reported to improve after IL resection in conjunction with local IL cryotherapy [11]. IL TA injection clinically and principally induces keloid regression by reducing the extracellular matrixŌĆÖs components; namely, collagen I, collagen III, elastin, and fibronectin. It also inhibits the proliferation of normal and keloid fibroblastsŌĆÖ and collagen synthesis, increases collagenase production, and reduces collagenase inhibitor levels [12].
Herein, we present the clinical application of the CryoPen for keloids in combination with IL TA injections as adjuvant therapy after surgical excision.
Among all patients who visited our scar laser center between January 2016 and August 2017, 528 patients received extralesional (EL) cryotherapy with the CryoPen and simultaneous IL TA injections for keloids. A chart review was performed for these patients. Patient data were obtained after acquiring written informed consent and following a protocol approved by the institutional board (IRB No. 2021-4625-001). The study was performed in accordance with the tenets of the Declaration of Helsinki of 1975.
The inclusion criteria were as follows: (1) having undergone initial IL keloid excision with EL cryotherapy and IL TA injections as adjuvant therapy; (2) age Ōēź19 years; and (3) provision of informed consent to photography and data usage for research purposes. The exclusion criteria were (1) age <19 years and (2) unwillingness to consent for research for reasons such as language problems (e.g., foreign patients).
Indications for excision surgery were a history of recurrence, existing symptoms, and bulky cases. All excisions were performed by a single surgeon. For EL cryotherapy, all procedures were performed using a portable, spray-type CryoPen (L&C BIO). Disposable nitrous oxide cartridges weigh 8 g, and the maximum spraying time per cartridge bottle is 120 seconds. A disposable nitrous oxide cartridge and filter are included in one set. After mounting the cartridge and filter in the device, when we press the spray button at a distance of 1 cm from the center of the keloid lesion, nitrous oxide at ŌĆō79 ┬░C is immediately sprayed and cryotherapy is performed. Anesthesia was not performed because the treatment took no longer than a few seconds and caused little pain. In the case of skin abrasion after cryotherapy, conservative treatment was concurrently performed and cryotherapy was postponed to the next time. The solutionŌĆÖs concentration for IL TA injections was 10ŌĆō40 mg/mL. The follow-up interval was 3 weeks and was increased from 3 to 6 months during the later part of treatment.
As a measure of the final outcome judgments before and after treatment, the Vancouver Scar Scale (VSS) score was calculated by retrospectively analyzing lesion images from photographs in the outpatient clinic.
In total, 53 patients with 54 keloid lesions were included in this retrospective interventional study (Table 1). There were 41 women and 12 men, and the average age of the patients was 34 years. There were two keloid lesions in a woman. The lesion was located in the head and neck region in 21 cases, the trunk in 20 cases, and the extremities in 13 cases. The most common causes of keloids were postoperative scars, followed by piercing, trauma, acne, vaccines, and burns. The average length of the keloids was 6.14 cm.
Among the 54 instances of keloid lesions treated with 4.42 cycles (range, 1ŌĆō11 cycles) of cryotherapy and IL TA injections, 31 cases maintained flatness without recurrence of the remnant keloid after surgery. In the remaining 23 cases, locally palpable lesions in the residual keloids were observed during treatment. For this focal relapsed remnant lesion, approximately four cycles of additional adjuvant therapy were administered. On average, the total number of sessions after surgery for all patients was 6.26. Among the 23 cases considered to have local regrowth, 18 cases (78.3%) were relieved during the additional treatment period, and no specific symptoms were observed. Five cases (9.3%) experienced relapse even after surgery and additional adjuvant treatment. Reoperation was performed for one of these patients.
Of the 54 patients, the VSS score was calculated for 45 patients with available photographs from both the start and end of treatment. The mean value at the start of treatment was 10.1 (range, 7ŌĆō13) and that at the end was 5.0 (range, 1ŌĆō8). Considering the specific items of the VSS, vascularity improved from 2 to 1.1, pigmentation from 1.9 to 1.6, pliability from 3.8 to 1.3, and height from 2.4 to 1 after treatment.
In 17 patients (31.5%), symptoms including pain, tenderness, erythema, itching, hardness, and pigmentation were observed after surgery, but these symptoms were controlled and maintained through adjuvant therapy.
In one patient, delayed healing and wound dehiscence occurred after keloidectomy and three sessions of adjuvant therapy of the anterior chest wall. After 6 months of conservative treatment, the wound healed, and cryotherapy was restarted with satisfactory results. Skin abrasion that occurred immediately after treatment was resolved with conservative treatments such as hydrocolloid agents or self-dressing with antibiotic ointment, and based on the chart review, there were no cases of delay or discontinuation of the next treatment session attributed to this reason. In addition, there were no cases of hypopigmentation, necrosis, abscess formation, blistering, or infection due to adjuvant therapy. The following sections present three cases of excision.
A 59-year-old woman visited our hospital with a keloid that appeared following abdominal surgery performed 5 years ago (Fig. 1A). First, the keloid was excised. After all the wounds had healed, nine cryotherapy sessions were performed, combined with steroid therapy, at least 3 weeks apart; thereafter, the patient was followed up for 12 months. Fig. 1B and C are images of the area taken during 6- and 12-month postoperative follow-up visits, respectively. She complained of severe itching and pain in the keloid area before surgery; however, the symptoms improved after the first session of combined treatment. Both the physician and patient were satisfied with the symptom improvement and final scar appearance.
A 32-year-old woman presented with a keloid on the left shoulder caused by inoculation 20 years ago (Fig. 2A). No previous treatments had been administered. Similar to patient 1, IL keloid excision was performed first. Fig. 2B is the image of the area taken immediately after surgery. After wound healing, nine sessions of cryotherapy were performed, combined with steroid therapy, at least 3 weeks apart. The patient was followed up for 8 months. Fig. 2C and D are images of the area from 3- and 8-month postoperative follow-up visits, respectively. Despite the redness of the scar, the physician and patient were satisfied with the final scar appearance.
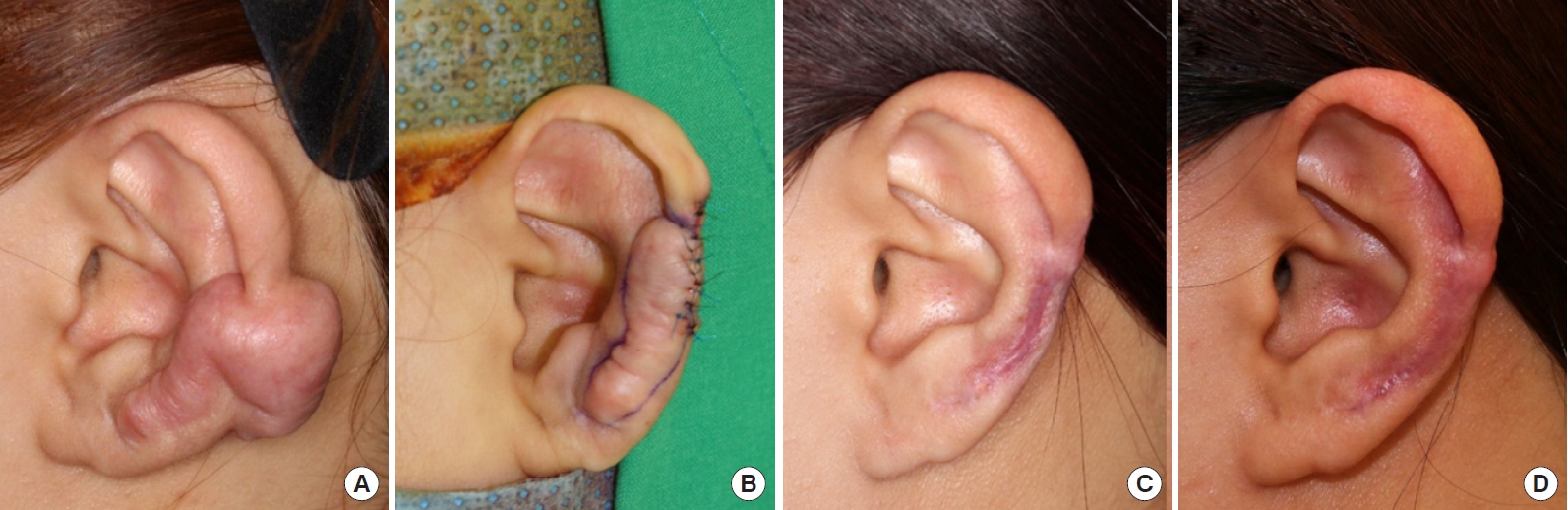
A 22-year-old woman presented with a keloid of the left helix caused by a piercing 5 years ago (Fig. 3A). Keloid excision had been performed twice at another hospital; however, the lesion recurred. First, an IL keloid excision was performed. Fig. 3B is an image of the area taken immediately after surgery. After all the wounds had healed, nine sessions of cryotherapy were performed, combined with steroid therapy, at least 4 weeks apart. After 36 months of follow-up, the results were found to be satisfactory by the physician and patient. Fig. 3C and D are images of the area taken at 9- and 24-month postoperative follow-up visits, respectively.
Globally, many studies have discovered monotherapies for keloids. This has led to a change of thought regarding combinations of multiple treatments in cases wherein one treatment has limited effectiveness.
The reason for hesitating to perform surgical resection is that it is an invasive act; thus, there is a limit to what a dermatologist can perform. Moreover, the operating surgeon is likely to have concerns regarding the high recurrence rate [13]. When only surgical resection of keloids was performed, the recurrence rates were reported to be close to 80% [14], 65%ŌĆō99% [15], or 45%ŌĆō100% [16]. Nevertheless, resection should be considered; it is thought that by removing the proliferative area at the center of the keloid through resection, it may be possible to reduce the proliferative power of the keloid, while rapidly reducing its volume [17].
In the case of large, bulky, or recurrent keloids, surgical excision should be considered. Staged excision should be considered if blood circulation in the surrounding tissues is likely to decrease after excision, if there is a possibility that the skin will be insufficient, or that tension may occur due to locational characteristics. Rather than trying to solve everything simultaneously, it is also important to reduce the patientŌĆÖs anxiety regarding their results by reminding them from the outset that treatment will take place over several sessions.
Cryotherapy using the CryoPen not only physically damages blood vessels and destroys tissues, but also increases CD163+ M2 macrophages and matrix metalloproteinase-9, which is molecularly and biologically involved in fibrotic resolution. Nevertheless, since the CryoPen has a freezing point of ŌĆō79 ┬░C, it can reduce side effects by enabling selective treatment compared to the traditional cryogun method [18].
In our study, recurrence after surgery was observed in 23 out of 54 patients, which is not significantly different from the results previously published in the literature. Recurrence after IL keloidectomy was considered impossible to prevent completely, as there would be a residual unresected keloid. If the size of the keloid after surgery is similar to the size before surgery, reoperation should be considered. If there is a focal or partial relapse that has not increased to the size of the previous keloid, the lesion is considered to be responsive to adjuvant therapy, and an average of six cycles should be performed and the progress should be monitored. Excluding two cases where treatment was stopped due to transfer to other hospitals, the final recurrence rate was three out of 52 cases (5.8%), which is a satisfactory result.
TA injections, when used alone, were found to be effective as the first-line treatment for keloids; however, studies on combination therapy are being actively conducted because of the possibility of recurrence or side effects. According to Yosipovitch et al. [19], the treatment effect was reportedly better when TA and cryotherapy were combined than when TA was used alone. The authors surgically excised the keloid first to create an environment that facilitates drug diffusion. When using cryotherapy-combined treatment, the concentration of TA can be adjusted to 10ŌĆō40 mg/mL according to the condition of the lesion; thus, the occurrence of side effects and recurrence rate are lowered due to the synergistic effect of these two methods.
The main limitation of this study is the absence of a control group. Additionally, the VSS for all cases could not be calculated because there were cases where photographs of patients were omitted, and there was no description of subjective indicators from the start to end of keloid treatment. Although the length of the keloid was measured in this study, measuring the volume reduction three-dimensionally would be more effective for proving the therapeutic effect. In subsequent studies, it will be necessary to produce objective results through the calculation of various indicators along with the inclusion of a control group.
Keloids, irrespective of their size, are difficult to treat and are inherently prone to recurrence. Owing to the lack of a definitive monotherapy regimen, all treatment methods should be pursued within limits that are useful, safe, and acceptable to both physicians and patients. This is especially true in challenging cases with large or recurrent keloids. The current study showed that initial direct surgical excision, followed by combined treatment with EL cryotherapy and IL TA injections, was effective in the management of such challenging cases. Additionally, EL cryotherapy using the CryoPen was found to be superior to conventional IL cryotherapy, as its application is safer, easier, and faster. Still, further studies with larger samples and long-term follow-up are required.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Severance Hospital (IRB No. 2021-4625-001) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and use of their images.
Fig.┬Ā1.
A 59-year-old woman presented with abdominal keloids due to postoperative scarring. Images of the lesion before treatment (A), at a 6-month follow-up (B), and at a 1-year follow-up (C).
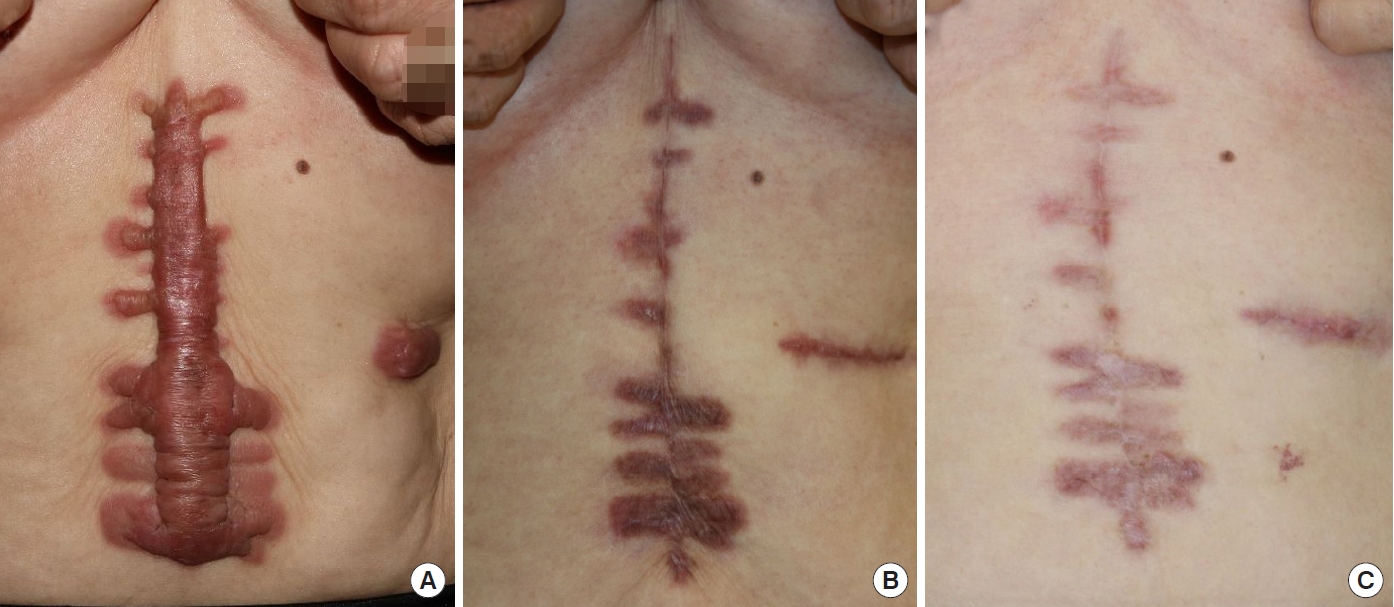
Fig.┬Ā2.
A 32-year-old woman presented with a left shoulder keloid secondary to vaccination. Images of the lesion before treatment (A), after partial keloid excision (B), at a 3-month follow-up (C), and at an 8-month follow-up (D).

Fig.┬Ā3.
A 22-year-old woman presented with a left ear keloid arising from a helix piercing. Images of the lesion before treatment (A), after partial keloid excision (B), at a 9-month follow-up (C), and at a 2-year follow-up (D).
Table┬Ā1.
Patient demographics
REFERENCES
1. Bailey JN, Waite AE, Clayton WJ, et al. Application of topical mitomycin C to the base of shave-removed keloid scars to prevent their recurrence. Br J Dermatol 2007;156:682-6.


2. Weshahy AH. Intralesional cryosurgery: a new technique using cryoneedles. J Dermatol Surg Oncol 1993;19:123-6.


3. Zouboulis CC, Orfanos CE. Cryosurgical treatment. In: Harahap M, editor. Surgical treatments for cutaneous scar revision. Marcel Deckker Inc.; 2000. p. 185-234.
4. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg 2003;111:1841-52.


5. Har-Shai Y. Intralesional cryosurgery for enhancing the involution of hypertrophic scars and keloid: a new fundamental adjunctive wound healing therapy based on experimental and clinical data. Cryobiology 2013;66:345-6.

6. OŌĆÖBoyle CP, Shayan-Arani H, Hamada MW. Intralesional cryotherapy for hypertrophic scars and keloids: a review. Scars Burn Heal 2017;3:2059513117702162.


7. van Leeuwen MC, Bulstra AE, Ket JC, et al. Intralesional cryotherapy for the treatment of keloid scars: evaluating effectiveness. Plast Reconstr Surg Glob Open 2015;3:e437.


8. Lee YI, Kim J, Yang CE, et al. Combined therapeutic strategies for keloid treatment. Dermatol Surg 2019;45:802-10.


9. Mourad B, Elfar N, Elsheikh S. Spray versus intralesional cryotherapy for keloids. J Dermatolog Treat 2016;27:264-9.


10. Park TH, Cho HJ, Lee JW, et al. Could -79 ┬░C spray-type cryotherapy be an effective monotherapy for the treatment of keloid? Int J Mol Sci 2017;18:2536.



11. Reissis D, Tickunas T, Agha RA, et al. Intralesional excision with topical intralesional cryotherapy improves the treatment of keloid scarring in a paediatric patient. Ann R Coll Surg Engl 2017;99:e233-335.



12. Niessen FB, Spauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 1999;104:1435-58.


14. Jfri A, Rajeh N, Karkashan E. A case of multiple spontaneous keloid scars. Case Rep Dermatol 2015;7:156-60.




15. Sclafani AP, Gordon L, Chadha M, et al. Prevention of earlobe keloid recurrence with postoperative corticosteroid injections versus radiation therapy: a randomized, prospective study and review of the literature. Dermatol Surg 1996;22:569-74.


16. Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg 2002;110:560-71.


17. Luo S, Benathan M, Raffoul W, et al. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg 2001;107:87-96.









