 |
 |
- Search
| Arch Aesthetic Plast Surg > Volume 29(2); 2023 > Article |
|
Abstract
Background
Immediate breast reconstruction after mastectomy can be challenging in some patients for medical or oncological reasons. Delayed two-stage tissue expander/implant breast reconstruction is a reliable option for these patients. However, data regarding surgical techniques, outcomes, and complication rates are limited. This study reports our experience using the two-stage tissue expander/implant procedure for delayed breast reconstruction.
Methods
This retrospective study included 32 patients (34 breasts) who underwent delayed two-stage tissue expander/implant breast reconstruction at our institution from January 2018 to July 2022. We summarized the techniques used in the procedure and evaluated the 1-year postoperative outcomes and complication rates.
Results
The mean time from mastectomy to expander insertion was 210±25 days, and 8.2±2.3 additional expansions were required prior to the implant insertion. The mean time of tissue expansion was 187±15 days, and the mean volume of expansion was 495±31 mL. No major complications occurred that required reoperation, and the patients were highly satisfied with the surgical results.
Conclusions
Although delayed two-stage tissue expander/implant breast reconstruction resulted in satisfactory outcomes, consensus regarding the operative technique is still needed. Two-stage tissue expander/implant breast reconstruction is a safe and effective option for delayed breast reconstruction.
The increasing frequency of mastectomy in patients with breast cancer is due to the increasing incidence of breast cancer and advances in imaging technology and genetic testing [1]. Several studies have reported that breast reconstruction after mastectomy positively affects patients’ quality of life and improves their body image [2]. As a result, the rate of immediate breast reconstruction after mastectomy is increasing, and surgical advances such as skin-sparing mastectomy have enabled prosthetic reconstruction using tissue expanders and implants [3]. The advantages of immediate breast reconstruction postmastectomy include positive cosmetic results, low costs, and improved psychosocial well-being. However, many surgeons recommend delayed breast reconstruction surgery for patients with significant medical comorbidities, ongoing postmastectomy radiation therapy, or oncological problems such as inflammatory breast cancer. Delayed breast reconstruction is also preferable when the patient has difficulty accepting the burden of immediate breast reconstruction surgery and is uncertain regarding adjuvant therapy [4].
Latissimus dorsi and transverse rectus abdominis myocutaneous(TRAM) flaps are commonly used in delayed breast reconstruction with autologous tissue [5]. However, breast reconstruction using autologous tissue has the potential for volume loss due to fat resorption in the flaps, muscle atrophy after surgery, donor site complications such as seroma, as well as long periods of scarring, long operation times, and long periods of hospitalization. In addition, the perfection of microsurgical techniques is still needed for procedures using free flaps such as free TRAM flaps [5,6]. Therefore, patients may prefer delayed two-stage tissue expander/implant breast reconstruction as an alternative, since there are no donor site complications, the operation and hospitalization times are shorter, and no additional scars are formed. Delayed two-stage tissue expander/implant breast reconstruction yields satisfactory esthetic results and leads to fewer postoperative complications [6].
However, consensus has not been reached on the ideal surgical technique to use for delayed two-stage tissue expander/implant breast reconstruction. Criteria such as placement of the tissue expander in relationship to the inframammary fold (IMF) line, determination of the tissue expander pocket and range, and the technique for setting the IMF line and forming the final shape of the IMF still vary [7,8]. Therefore, this study presents an efficient and safe approach by introducing operative techniques and evaluating the outcomes and complications of patients who underwent delayed two-stage tissue expander/implant breast reconstruction at a single institution.
This retrospective study included data from 32 patients (34 breasts) who underwent delayed two-stage tissue expander/implant breast reconstruction at a single institution from January 2018 to July 2022. All operations were performed by a single plastic surgeon. Patients aged 30 to 60 years who were diagnosed with breast cancer and underwent delayed breast reconstruction using tissue expanders/implants were included in this study. The pectoralis major muscle was preserved during the initial mastectomy in all patients. The delayed reconstruction occurred at least 1 year after the completion of oncological treatments to ensure the improved quality of scar tissue and the surgical site. Patients with severe diabetes mellitus and those considered to be heavy smokers were excluded from the study. All procedures were carried out in accordance with the standards of the Ethics Committee and the Institutional Review Board of the hospital (No. 2021-06-025) and all patients provided informed consent to have their data (including anonymized photographs) recorded, analyzed, and published for research purposes. The study was conducted according to the Declaration of Helsinki.
The existing second breast was used as a model and preoperative taping was used to measure its volume. Breast volumetric analysis with a Vectra H2 three-dimensional scanner (Canfield Scientific) was used for esthetic planning. Baseline measurements of the breast (width, height, projection, and skin thickness) were performed by a single surgeon, and the appropriate tissue expander was selected. In patients who underwent bilateral mastectomy, the appropriate tissue expander was determined based on the patient’s preferred breast volume, body mass index (BMI), and skin thickness. During the second stage, the most appropriate implant was selected based on the final breast volume, contour, size, symmetry, and skin thickness using an implant sizer to ensure breast symmetry.
To position the tissue expander during the first stage of reconstruction, a new IMF line was designed 2 cm below the patient’s normal IMF (Fig. 1A and B). Once the preexisting scar was excised, the skin flaps were elevated to expose the lower margin of the pectoralis major muscle. Following the incision line, we carefully dissected the skin flap overlying the pectoralis major muscle, being careful not to create excessive tension on the skin flap and monitoring the color of the skin. Medial superior dissection was performed from the lateral border to the bottom of the pectoralis major muscle to create a musculofascial pocket, into which the tissue expander was inserted. In addition, lateral dissection was performed below the serratus muscle and/or the overlying fascia. Inferiorly, the rectus fascia was raised to become continuous with the serratus fascia along the new IMF line. In this way, the tissue expander was placed 2 cm below the patient’s IMF line (Fig. 2A). Finally, a fasciotomy was performed through the inferolateral fascia at or just below the IMF to facilitate flap expansion and avoid displacement of the tissue expander [8]. This technique created adequate space to place the tissue expander without causing it to fold (Fig. 2B).
The surgical field was then irrigated using an antibiotic irrigation solution (povidone-iodine, 50 mL; isepamicin, 80 mg; and cefazolin, 1 g in 500 mL of sterile saline). After the tissue expander was carefully inserted into the pocket, fixation sutures using 3-0 Ethibond (Ethicon Ltd.) were used to bind the lateral and medial parts of the tissue expander to the muscle tissue to ensure the correct fit and fixation of the implant in the breast pocket. Negative pressure drains were positioned in the submuscular layer. The tissue expander was inflated to approximately 10% to 20% of the total volume. The surgical field was closed layer-by-layer using 2-0 Vicryl, 4-0 Vicryl, and 5-0 Ethilon sutures (Ethicon Ltd.).
Although the standard regimen for serial tissue expander inflation recommends the infusion of 50 to 100 mL of saline every week, the intervals and volumes of inflation were determined on a patient-by-patient basis.
The second stage of reconstruction was conducted when the desired volume of the expander was reached. The position of the expanded tissue was corrected to mirror the existing IMF line by advancing the IMF line upward (Fig. 1C and D). The tissue expander was removed and the IMF was set using 2-0 Ethibond sutures (Ethicon Ltd.) to fix the deep dermis to the anterior chest wall (Fig. 2C and D). The fold was reconstructed using several sutures, creating an esthetically pleasing inferolateral curve. The surgical field was irrigated using the same antibiotic solution used in the first stage. A sizer was used to determine the appropriate implant, which was then inserted into the pocket. The appearance and symmetry of the implant with the contralateral breast were verified with the patient in the sitting position. Finally, the surgical field was closed using 2-0 Vicryl, 4-0 Vicryl, and 5-0 Ethilon sutures (Ethicon Ltd.).
Data regarding patient demographics, the time from mastectomy to expander insertion (days), duration of tissue expansion (days), hospital stay (days), size of the expander (mL), volume of expansion (mL), number of additional expansions, use of acellular dermal matrix, subsequent operations (including fat injections and contralateral augmentation), and radiation therapy were collected from patients’ medical charts. All patients were evaluated for complications, including flap necrosis, seroma, hematoma, infection, expander/implant salvage, and capsular contracture up to 12 months after the second stage of implant insertion. The evaluation was performed by direct physical examination, observation of photographs, and monitoring of magnetic resonance images. In this study, seroma was defined as a fluid collection occurring 30 days after drain removal. Skin flap necrosis was defined as a partial- to total-thickness ischemic tissue change, with or without debridement. Capsular contracture was diagnosed in patients with Baker classification grade 3 or 4 contracture. Infections were diagnosed in patients with erythema or in those who were treated with antibiotics.
The Kyungpook National University Hospital (KNUH) modification of the Breast-Q, a satisfaction questionnaire, was completed by each patient 12 months after the second stage of surgery (Table 1). The Modified KNUH Breast-Q evaluated patients’ overall satisfaction and satisfaction with symmetry, size, shape, postoperative pain, scarring, postoperative confidence, and sense of sexual attractiveness, with a total score of 55. Higher scores indicated higher satisfaction [9].
The mean patient age at the time of the expander insertion (first stage) was 50.2±7.8 years, and the mean BMI was 22.5±2.9 kg/m2 (Table 2). The mean time from mastectomy to expander insertion was 210±25 days, and 8.2±2.3 additional expansions were required before implant insertion. The mean duration of tissue expansion was 187±15 days, and the mean volume of expansion was 495±31 mL. An acellular dermal matrix was used in seven patients (21%), contralateral augmentation was performed in four patients (12%), and fat injections were used in two patients (6%) to achieve cosmetic satisfaction.
Four patients (12%) experienced flap necrosis and six (18%) experienced seroma (Table 3). Flap necrosis was treated with dressings, and the seromas resolved within 1 month after simple aspiration. No infections, hematomas, tissue expander or implant loss, or capsular contracture occurred. The average satisfaction score on the KNUH-modified Breast-Q was 48.7/55 (Table 4).
A 37-year-old female patient with a contralateral (left) breast volume of 260 cc and a BMI of 23.4 kg/m2 underwent delayed two-stage breast reconstruction. The patient underwent modified radical mastectomy for (right) breast cancer in May 2016. In December 2018, the patient underwent delayed reconstruction with tissue expander insertion (450 cc). No acellular dermal matrix was used. The total volume of expansion was 510 cc, and 6 months after the first stage of surgery, the patient underwent delayed reconstruction with a silicone implant insertion (250 cc). Six months after the second stage of surgery, nipple reconstruction was conducted using a C-V flap. No complications were observed 1 year after surgery, and the patient was satisfied with the surgical results (Fig. 3).
A 58-year-old female patient with a BMI of 22.6 kg/m2 underwent delayed two-stage breast reconstruction. The patient underwent bilateral modified radical mastectomy with axillary lymph node dissection for breast cancer in November 2002. In August 2018, a tissue expander (550 cc) was inserted on both sides with an acellular dermal matrix (4×12 cm) inserted into each breast. The volume of expansion was 530 cc for each breast. In February 2019, the patient underwent delayed reconstruction with silicone implant insertion (400 cc). Six months after the second stage of surgery, nipple reconstruction was conducted using a C-V flap. There were no complications 1 year after surgery, and the patient was satisfied with the surgical results (Fig. 4).
The two methods of breast reconstruction after mastectomy involve either autologous tissue or implants. The use of implants for reconstruction has increased for several reasons, including patient and operator preferences, changes in oncologic practice, and an increased incidence of bilateral mastectomies [1]. Direct-to-implant reconstruction quickly corrects the shape of the breast, is cost-effective, and improves patients’ psychological well-being. However, delayed reconstruction is recommended in some patients for medical or oncological reasons [3,4,10,11], and the delayed tissue expander/implant two-stage breast reconstruction method is commonly performed [4,7,12].
Several studies have confirmed the efficacy and safety of two-stage tissue expander/implant breast reconstruction [4,7,8,11-14]. However, various techniques are used based on the surgeon’s preference, and no consensus on the ideal technique has been reached.
Defining the pocket boundary and positioning the IMF are the most important steps for maintaining breast symmetry during insertion of the tissue expander [8,12]. Anatomical expanders were initially used to position the tissue expander at the level of the IMF, to maintain the shape and position of the tissue expander and the contour of the IMF during expansion, while preserving the IMF line [15]. However, the maximum expansion point of the tissue expander is ideal when it is located in the lower third of the breast, and when the tissue expander is placed at the IMF line, the maximum expansion point is too high and the shape of the lower pole of the breast may be constrictive [8]. Additionally, as the volume of the tissue expander increases, the chest tissue around the expander may tighten. To address both issues, the tissue expander should be placed 1 to 2 cm inferior to the IMF line [8,16,17]. Therefore, the tissue expander was placed 2 cm inferior to the IMF line at our hospital, so that the area of maximum expansion would be in the lower third of the breast, resulting in an ideal breast contour postoperatively. The reconstructed breast should have a natural and ptotic look. When the expander is placed 2 cm inferior to the IMF line, the tissues in the abdominal direction naturally advance, allowing natural esthetic outcomes based on the neo-IMF line formed after the second stage of reconstruction. However, the band of fascia around the lower pole of the breast may limit the expansion. To overcome this limitation, fasciotomy can be performed on the rectus fascia in the inferomedial direction and on the serratus fascia of the lateral side. Excellent cosmetic results were achieved when the tissue expander was placed in the appropriate position.
Muscle is traditionally used to position the tissue expander and implant pocket [18]. Pusic and Cordeiro [19] reported total muscle coverage via the dissection of the serratus muscle and fascia from the lateral side. Disa et al. [20] dissected and raised the pectoralis major and the serratus muscle for muscle coverage. However, although dissection of the pectoralis major is not challenging and results in minimal trauma, when the serratus muscle is completely dissected from the rib, postoperative pain occurs, and an ideal contour of the inferolateral breast is not maintained [8]. The musculofascial pocket, which uses the entire pectoralis muscle for the superior portion, but only a part of the serratus muscle and the overlying fascia for the lateral portion, is an alternative reconstruction method. The musculofascial pocket is used during tissue expansion at our hospital to achieve the ideal contour since the risk of the tissue expander folding during expansion is reduced. In addition, less postoperative pain is reported during expansion when this method is used.
In this study, acellular dermal matrix was used in seven patients (21%). Rawlani et al. [21] suggested that suturing acellular dermal matrix to the serratus fascia increases the tissue expander volume, forming a larger and more spacious pocket, which is otherwise restricted by the pectoralis muscle. Therefore, acellular dermal matrix was utilized in our patients to secure a stable pocket when the overlying fascia was insufficient to form a musculofascial pocket. This method also minimized postoperative complications when used during the second stage (implant insertion), and resulted in high patient satisfaction with the cosmetic outcomes.
In this study, six patients (18%) underwent delayed two-stage tissue expander/implant breast reconstruction after receiving radiation therapy. Terao et al. [22] investigated postmastectomy radiation therapy (PMRT) and the condition of the irradiated chest skin of the flap, and recommended avoiding PMRT because of the poor blood supply, flap necrosis, and scar contracture due to radiation damage. However, Seth et al. [4] compared and analyzed delayed and immediate tissue expander reconstruction. They reported a low incidence of complications in the delayed tissue expander reconstruction group after PMRT [4]. In patients who have undergone radiation therapy, tissue expander reconstruction can be difficult because of the poor skin flap condition. However, if enough time elapses after PMRT, the condition of the skin flap and blood supply can stabilize and delayed reconstruction is possible [4]. In this study, major complications requiring implant removal after surgery were not found, most likely because delayed reconstruction provided time for the skin flaps to heal after radiation therapy, and complications were minimized during expansion by providing an adequate musculofascial pocket in the first-stage operation. Although there were some complications in this study, including two seromas and one flap necrosis among the patients who underwent breast reconstruction after PMRT, no major complications occurred. We selected patients who had sufficient time after PMRT and adequate skin flaps. In patients who have undergone PMRT, the degree of pain, skin color, and other complications during expansion should be observed closely, and regular follow-up is needed after surgery.
Fat injection and contralateral augmentation were conducted in 6% and 12% of patients, respectively. Fat grafts are widely used as soft tissue supplements due to their convenience and stability [23]. Delay et al. [24] reported that fat grafting was a major factor influencing the development of prosthetic breast surgery. Autologous fat grafts are widely used for autologous and implant-based breast reconstruction as they improve breast deformities and correct asymmetry [23]. Therefore, autologous fat graft injections were used in this study to achieve bilateral symmetry during the second stage of implant insertion. However, to obtain excellent cosmetic results bilaterally, contralateral augmentation was conducted when sufficient volume could not be obtained via an autologous fat graft alone, ultimately leading to high patient satisfaction. Autologous fat grafting and contralateral augmentation are used to add volume and correct symmetry during breast reconstruction.
Complications of tissue expander/implant breast reconstruction include infection, skin necrosis, seroma, capsular contracture, and tissue expander/implant extrusion. In this study, seromas formed in 18% of patients and flap necrosis occurred in 12% of patients. However, these complications resolved after simple aspiration or dressing. No significant complications requiring expander/implant salvage or reoperation occurred in this study. The lack of serious complications may be due to the appropriate positioning of the tissue expander.
This study was not without limitations. Autologous reconstruction and two-stage tissue expander/implant breast reconstruction are the most commonly used methods for delayed reconstruction. Therefore, analysis of a larger patient group, including patients who undergo breast reconstruction using a wider variety of methods, is needed.
In conclusion, the techniques used to achieve delayed two-stage tissue expander/implant breast reconstruction at our hospital resulted in favorable outcomes, few complications, and high patient satisfaction. However, guidelines regarding the most effective techniques to achieve delayed two-stage tissue expander/implant breast reconstruction should be established.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Kyungpook National University Medical Center (IRB No. 2021-06-025) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
The patients provided written informed consent for the publication and use of their images.
Fig. 1.
Tissue expander placement and preoperative design for two-stage breast reconstruction. (A) The patient’s preoperative condition. (B) The first-stage design, including creation of a new inframammary fold (IMF) line 2 to 3 cm below the patient’s normal IMF. (C) The design of the second stage. (D) The patient is shown 1 year after completion of the second stage.
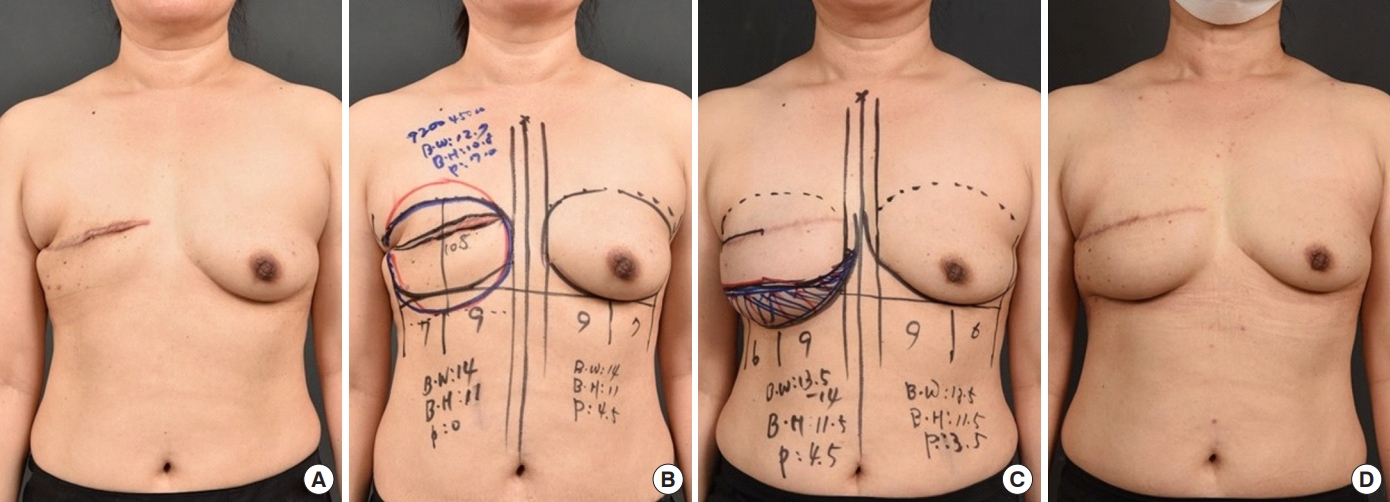
Fig. 2.
Delayed tissue expander/implant two-stage breast reconstruction after mastectomy. (A) During the first stage of tissue expander reconstruction, the subpectoralis major, subrectus fascia, and subserratus anterior plane are dissected. (B) A new inframammary fold (IMF) line is created 2 to 3 cm inferior to the original IMF line. The tissue expander is inserted at the new IMF line. (C) The expanded tissue is 2 to 3 cm inferior to the IMF line during the second stage of implant reconstruction. (D) The new IMF line is made by advancing the expanded tissue upwards.

Fig. 3.
Delayed tissue expander/implant two-stage breast reconstruction results. A 40-year-old woman with right breast cancer underwent mastectomy and delayed tissue expander/implant two-stage breast reconstruction. (A) The patient is shown before the first stage of tissue expander reconstruction. (B) The patient is shown between the first and second stages of reconstruction surgery. (C) The patient is shown 1 year after the second stage of reconstruction and tattoo.
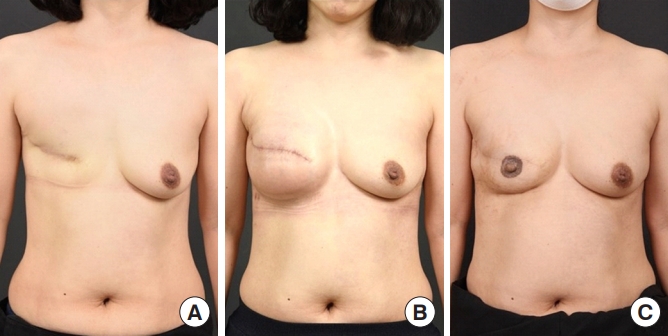
Fig. 4.
Delayed tissue expander/implant two-stage breast reconstruction results. A 58-year-old woman with bilateral breast cancer underwent mastectomy and delayed tissue expander/implant two-stage breast reconstruction. (A) The patient before the first stage of tissue expander reconstruction. (B) The patient just prior to the completion of the second stage of surgery. (C) The patient is shown 1 year after the second stage of surgery and nipple reconstruction with a C-V flap.
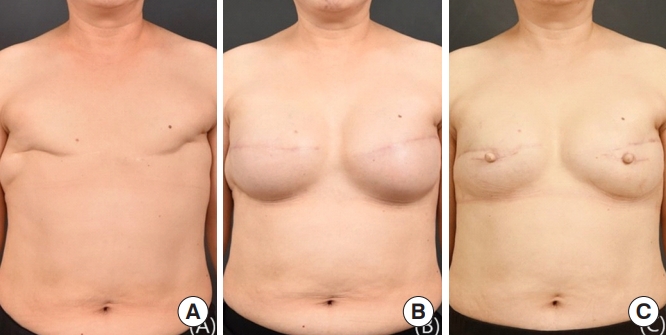
Table 1.
The Kyungpook National University Hospital (KNUH) modification of the Breast-Q
Table 2.
Patient demographics and breast reconstruction outcomes
REFERENCES
1. Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg 2013;131:15-23.

2. Yoon AP, Qi J, Brown DL, et al. Outcomes of immediate versus delayed breast reconstruction: results of a multicenter prospective study. Breast 2018;37:72-9.



3. Azouz V, Lopez S, Wagner DS. Surgeon-controlled comparison of direct-to-implant and 2-stage tissue expander-implant immediate breast reconstruction outcomes. Ann Plast Surg 2018;80:212-6.


4. Seth AK, Silver HR, Hirsch EM, et al. Comparison of delayed and immediate tissue expander breast reconstruction in the setting of postmastectomy radiation therapy. Ann Plast Surg 2015;75:503-7.


5. Demiri EC, Tsimponis A, Pagkalos A, et al. Fat-augmented latissimus dorsi versus deep inferior epigastric perforator flap: comparative study in delayed autologous breast reconstruction. J Reconstr Microsurg 2021;37:208-15.


6. Rykała J, Szychta P, Kruk-Jeromin J. Delayed two-stage breast reconstruction with implants: the authors’ recent experience. Can J Plast Surg 2011;19:88-92.




7. Dikmans RE, Negenborn VL, Bouman MB, et al. Two-stage implant-based breast reconstruction compared with immediate one-stage implant-based breast reconstruction augmented with an acellular dermal matrix: an open-label, phase 4, multicentre, randomised, controlled trial. Lancet Oncol 2017;18:251-8.


8. Cordeiro PG, Jazayeri L. Two-stage implant-based breast reconstruction: an evolution of the conceptual and technical approach over a two-decade period. Plast Reconstr Surg 2016;138:1-11.



9. Kim JB, Eom JR, Lee JW, et al. Utility of two surgical techniques using a lateral intercostal artery perforator flap after breast-conserving surgery: a single-center retrospective study. Plast Reconstr Surg 2019;143:477e-487e.


10. Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2000;106:1014-27.


11. Susarla SM, Ganske I, Helliwell L, et al. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast Reconstr Surg 2015;135:1e-8e.


12. Bellini E, Pesce M, Santi P, et al. Two-stage tissue-expander breast reconstruction: a focus on the surgical technique. Biomed Res Int 2017;2017:1791546.




13. Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer 2012;118:2552-9.


14. Pallara T, Cagli B, Fortunato L, et al. Direct-to-implant and 2-stage breast reconstruction after nipple sparing mastectomy: results of a retrospective comparison. Ann Plast Surg 2019;83:392-5.


15. Maxwell GP, Falcone PA. Eighty-four consecutive breast reconstructions using a textured silicone tissue expander. Plast Reconstr Surg 1992;89:1022-36.


16. Hammond DC. Technique and results using MemoryShape implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg 2014;Sep;134(3 Suppl): 16S-26S.


17. Spear SL, Spittler CJ. Breast reconstruction with implants and expanders. Plast Reconstr Surg 2001;107:177-87.


18. Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 1981;67:312-7.

19. Pusic AL, Cordeiro PG. An accelerated approach to tissue expansion for breast reconstruction: experience with intraoperative and rapid postoperative expansion in 370 reconstructions. Plast Reconstr Surg 2003;111:1871-5.


20. Disa JJ, Ad-El DD, Cohen SM, et al. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg 1999;104:1662-5.


21. Rawlani V, Buck DW 2nd, Johnson SA, et al. Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann Plast Surg 2011;66:593-7.


22. Terao Y, Taniguchi K, Fujii M, et al. Postmastectomy radiation therapy and breast reconstruction with autologous tissue. Breast Cancer 2017;24:505-10.










