 |
 |
- Search
| Arch Aesthetic Plast Surg > Volume 29(3); 2023 > Article |
|
Abstract
Background
The longstanding and common use of hyaluronic acid (HA) has driven the expanded development of various commercial HA fillers. However, differences in the components of these HA fillers lead to variations in their effect. We compared the in vivo safety and efficacy of biphasic HA (BHA) and a new monophasic HA (MHA) for improving facial wrinkles. We investigated differences in outcomes after their injection into nasolabial folds (NLFs) using the Wrinkle Severity Rating Scale (WSRS), patient satisfaction using the Global Aesthetic Improvement Scale (GAIS), and pain using a visual analog scale (VAS). We also performed a safety assessment of the two fillers.
Methods
This matched-pair, double-blind, randomized study compared the degree of temporal wrinkle improvement in the NLFs of 91 participants using the BHA filler versus the new MHA filler. Safety and efficacy were compared at 8 and 24 weeks.
Results
At 24 weeks after application, the average WSRS scores were 2.17±0.72 (BHA) and 2.07±0.71 (MHA) (P=0.034). The average GAIS scores, as measured by a treating investigator at 8 weeks and 24 weeks, were 0.94±0.76 (BHA) and 0.98±0.78 (MHA) at 8 weeks (P=0.181), and 0.44±0.64 (BHA) and 0.49±0.69 (MHA) at 24 weeks (P=0.103). The VAS pain score was 0 points at 30 minutes after filler application in both groups.
In 1934, Karl Meyer and his assistant John Palmer discovered hyaluronic acid (HA), a high-molecular-weight biopolysaccharide, in the vitreous of bovine eyes. HA, a spontaneously occurring biopolymer, has significant biological functions in bacteria and higher animals, including humans. Although HA is present in most connective tissues, it is specifically concentrated in the synovial fluid, vitreous fluid of the eye, umbilical cords, and chicken combs. It is naturally synthesized by a class of integral membrane proteins called hyaluronan synthases and degraded by a family of enzymes called hyaluronidases [1].
HA demonstrates poor biomechanical characteristics when used as a dermal filler in its natural state. Although HA has excellent biocompatibility and affinity for water molecules, it is a soluble polymer that clears rapidly when injected into normal skin [2,3]. Chemical modification is required to improve the mechanical characteristics and residence time at the implant site. This allows HA to lift and improve skin wrinkles. HA contains abundant hydroxyl (alcohol) and carboxylic acid functional groups. Many cross-linking methods have been attempted to enhance the biomechanical characteristics of HA while retaining its biocompatibility and biological activity [4,5]. Biomaterials have been produced by modifying the carboxyl acid groups in HA by esterification and by using cross-linkers such as dialdehydes and disulfides [5].
One of the most important features of monophasic HA (MHA) fillers is that they have a homogeneous distribution of HA. Therefore, even the smallest space between the collagen and elastin fibrils can be filled with it. By contrast, biphasic HA (BHA) fillers are composed of numerous particles that form larger agglomerates, thereby shifting collagen and elastin fibers and leading to a less homogeneous HA distribution after the procedure. The difference arises from the cross-linking strategy, which makes the MHA fillers less elastic and more cohesive than the BHA fillers [6].
Although both filler types are widely used, their effects have not been compared. Both HA fillers can be used on various facial areas such as the forehead, glabella, perioral area, and nasolabial fold (NLF) [7]. However, the NLF is one of the most difficult areas for achieving patient satisfaction after treatment, and considerable caution is required because of the anatomical vulnerability of the site. Further quantitative studies are required to address these issues [8].
In the present study, we analyzed and compared the efficacy and safety of the BHA filler and the new MHA filler for NLF correction.
Patients aged 30 to 65 years with Wrinkle Severity Rating Scale (WSRS) scores of 3–4 (none, 1; shallow, 2; moderately deep, 3; very long and deep, 4; extreme, 5) and with visually symmetrical bilateral NLFs were selected for this study. Altogether, 94 patients enrolled in the study and three failed to meet the examination qualifications, leaving 91 participants in this study. Patients with a medical or drug history that could affect the study outcomes were excluded.
Participation was voluntary and patients were allowed to opt out at any time. All processes that involved human participants in this study were conducted in compliance with the ethical standards of our institution, the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. This study was approved by the Institutional Review Board of Nowon Eulji Medical Center (IRB No. 2018-10-004) and written informed consent was obtained from all participants. The study protocol conformed to the guidelines of the Declaration of Helsinki and to the Korean Good Clinical Practice guidelines.
The BHA filler used was Restylane LYFT with Lidocaine (Galderma Laboratories). The new MHA filler used in the study was Meline No. 3 with lidocaine (Bio Standard Inc.). Meline No. 3 with lidocaine is an injection gel made by adding and cross-linking a small amount of 1,4-butanediol diglycidyl ether (BDDE), a cross-linker, to sodium hyaluronate, which is made by fermenting and purifying Streptococcus bacteria. Both the BHA filler and the new MHA filler contained nonanimal-derived cross-linked HA and lidocaine. Their methods of use and mechanisms are similar to each other, and the indications for the use of these products matched our clinical investigation plan. Thus, these products were considered suitable for a comparative study.
To minimize differences in the depth or degree of the wrinkles in each subject, the surgical sites, the procedure characteristics (location, conditions, and duration), and the degree of recovery for each subject, a matched-pair design was adopted as the comparison method in this study. Thus, the BHA and MHA fillers were applied respectively to opposite sides of the bilateral NLFs in each subject. The filler application procedure was carried out by one treating investigator. Accordingly, 1.0 mL of each filler was injected using 27-gauge needles, respectively, into opposite sides of the bilateral NLFs of participants for this randomized, double-blind, and matched-pair trial design.
The WSRS and the Global Aesthetic Improvement Scale (GAIS) (5, very much improved; 4, much improved; 3, improved; 2, no change; 1, worse) were used to evaluate the clinical efficacy of the cosmetic outcomes. The WSRS scores were assessed by three independent evaluators (board-certified plastic surgeons) and a treating investigator using the filler-injection site photographs of the participants. The GAIS scores were measured by subject self-assessment and assessment by a treating investigator at 0, 2, 8, and 24 weeks.
A 100-mm visual analog scale (VAS) was used for pain evaluation, and the pain at each treatment site was evaluated independently at the end of the injection and at 15-minute intervals for 30 minutes after injection to evaluate pain reduction efficacy.
All adverse events (AEs), such as localized facial redness, swelling, pruritus, or skin induration, that occurred during this clinical trial were included in the safety evaluation. Laboratory tests and physical examinations conducted during the clinical trial period were also used to evaluate safety. All AEs were classified based on the System Organ Class of the World Health Organization-Adverse Reaction Terminology version 092.
Statistical analyses were performed using SAS version 9.1 or later (SAS Institute, Inc.). WSRS scores for the BHA filler and the new MHA filler, as assessed by the independent evaluators at 8 and 24 weeks, were compared using the paired t-test and the Wilcoxon signed-rank test. The proportion of participants in each group whose WSRS scores (as assessed by the independent evaluators and a treating investigator at 24 weeks after the final filler application) differed by at least 1 point from the baseline was compared using the McNemar test. To compare differences in percentages between the groups for the safety analysis and laboratory tests, we used the McNemar chi-square test. A 97.5% one-tailed confidence interval in the non-inferiority test was used to confirm the primary efficacy measure. For other difference comparisons, two-tailed tests at the 5% significance level were performed. Statistical significance was set at P < 0.05.
After screening for study eligibility, three out of 94 individuals who consented to participate in this clinical trial were excluded. The remaining 91 participants were randomized into groups that were injected with the BHA filler and the new MHA filler. After one withdrew their consent and dropped out of the study, a total of 90 participants completed this clinical trial in accordance with the protocol (Fig. 1). There were 14 men (15.4%) and 77 women (84.6%), with an average age of 50.26 ± 7.34 years.
The average WSRS scores for the BHA and new MHA fillers were assessed by independent evaluators at 8 weeks after final filler application, and the WSRS scores were 2.11 ± 0.72 and 2.01 ± 0.71 for the BHA and the MHA groups, respectively, with no significant intergroup differences (P = 0.055). However, at 24 weeks, the primary efficacy endpoint, the WSRS scores were 2.17 ± 0.72 and 2.07 ± 0.71 in the BHA and MHA groups, respectively, with the latter being significantly lower than the former (P = 0.034) (Table 1 and Figs. 2, 3).
The average WSRS scores at 8 weeks after filler application as assessed by a treating investigator were 2.73 ± 0.99 and 2.77 ± 0.97 in the BHA and the MHA groups, respectively, with no statistically significant difference between the two groups (P = 0.158). At 24 weeks after filler application, the average WSRS scores in the BHA and the MHA groups were 3.23 ± 0.91 and 3.27 ± 0.88, respectively, again showing no statistically significant difference between the two groups (P = 0.158).
The average GAIS scores, as assessed by a treating investigator, at 8 weeks after final filler application were 0.94 ± 0.76 and 0.98 ± 0.78 in the BHA and the MHA groups, respectively, presenting no statistically significant difference between the two groups (P = 0.181). At 24 weeks, the average GAIS scores were 0.44 ± 0.64 and 0.49 ± 0.69 for the BHA and the MHA groups, respectively, again showing no statistically significant intergroup differences (P = 0.103).
In addition, the average GAIS scores at 8 weeks after filler application, as assessed by the participants themselves, were 1.84 ± 0.76 and 1.92 ± 0.72 in the BHA and the MHA groups, respectively, showing no statistically significant difference between the two groups (P = 0.070). However, at 24 weeks, these scores were 1.31 ± 0.87 and 1.40 ± 0.86 in the BHA and the MHA groups, respectively; at this time point, the MHA group exhibited significantly higher average GAIS scores than the BHA group (P = 0.032).
The average VAS scores within 30 minutes after receiving the final applications of the BHA and new MHA fillers were compared. The BHA and MHA groups had average VAS scores of 1.67 ± 1.19 and 2.20 ± 1.44, respectively, immediately after filler application, with the BHA group showing a significantly lower average VAS score than the MHA group (P < 0.001). Conversely, 15 minutes after final filler application, the average VAS scores were 0.79 ± 1.22 and 0.00 ± 0.00 in the BHA and the MHA groups, respectively, with the BHA group showing a significantly higher average VAS score than the MHA group (P < 0.001). At 30 minutes after filler application, all participants in both groups showed VAS scores of 0.
Based on the independent evaluators’ assessment, the proportion of participants whose WSRS scores improved by > 1 point (efficacy improvement) at 24 weeks after final filler application (compared to their baseline scores) was 19.6% (nine participants) and 48.9% (22 participants) in the BHA and the MHA groups, respectively. Thus, the improvement rate in the MHA group was 2.44 times higher than that in the BHA group. In contrast, according to the treating investigator, three participants in the BHA group and one participant in the MHA group showed efficacy improvement, which was not a statistically significant difference.
Notably, there were no serious AEs. One of the five AEs occurred at a BHA application site, two at an MHA application site, and the remaining two at other areas where fillers had not been applied. The reported AEs included one case of partial skin necrosis, two cases of mild edema, one case of headache, and one case of chronic gastritis. The partial skin necrosis occurred at the injection site 3 days after injection with the BHA filler, along with a 0.1-cm sized skin discoloration at the right alar area of the nose. These areas resolved naturally within 10 days, without the application of hyaluronidase or antibiotic ointment.
The application of fillers to fill wrinkles has become a fundamental treatment in contemporary cosmetic procedures. Because of the increasing demand for filler injections, the market for dermal fillers has grown dramatically over the last several years [9]. HA has become the primary filler material for facial contouring via intradermal injections because of its many advantages over other filler agents [10].
HA dermal fillers typically fall into two categories (monophasic or biphasic) based on their variations in cross-linking [11]. MHA fillers are more cohesive than BHA fillers and can last longer without migrating significantly after application. However, BHA fillers are more easily customized to obtain the particle size suited to the indication and the anatomical area to be treated [12].
MHA fillers can be subdivided into monodensified and polydensified types. Monophasic monodensified gels are produced by mixing HAs and cross-linking in one step [11], whereas monophasic polydensified gels are generated by cross-linking HAs in an initial step, then a new amount of HA is added and additional cross-linking is performed [13].
According to a previous histological study [11], BHA fillers tend to agglomerate into large clumps without spreading evenly throughout the implanted tissue, whereas monophasic polydensified fillers spread evenly throughout the implanted tissue and seldom form clumps.
Monophasic monodensified fillers such as Meline No. 3 with lidocaine (Bio Standard Inc.) share the characteristics of both biphasic and monophasic polydensified fillers; therefore, they can exhibit clumping. Overall, however, they spread homogeneously throughout the implanted tissue. Many patients have reported that products in the monophasic monodensified gel family are less painful than those in the biphasic family, likely because of these histological differences [11]. Conversely, in this study, the VAS scores of the BHA group were significantly lower than those of the MHA group immediately after filler application. However, at 15 minutes the MHA group showed significantly lower VAS scores than the BHA group. Notably, at 30 minutes, all participants in both groups had VAS scores of 0.
The Meline No. 3 with lidocaine (Bio Standard Inc.) used in this study is a novel monodensified monophasic filler. It is chemically cross-linked with BDDE and is a nonanimal-based HA filler. Many types of chemical cross-links are used for HA dermal fillers, and BDDE is the most commonly used method. When the etherification reaction between the hydroxyl group of HA and the epoxy group of the cross-linking agent BDDE occurs, the conjugated HA gel that is subsequently formed is gradually decomposed by hyaluronidase and free radicals once it reaches the skin [14,15]. Restylane LYFT (Galderma Laboratories), which contains lidocaine, belongs to the BHA filler family. Meline No. 3 with lidocaine and Restylane LYFT have several similarities. Both are chemically cross-linked with BDDE and contain 0.3% lidocaine and a nonanimal-based HA filler.
The differences in the biophysical characteristics of MHA and BHA fillers are also clinically important. Compared with BHA fillers, the most important biophysical properties of MHA fillers are their low elasticity and high viscosity. The elastic modulus is a quantitative value of the gel stiffness and capacity to resist deformation under applied pressure. Viscosity refers to the ability of the gel to resist the shearing forces applied to the filler during and after injection. The term “tan delta” refers to the ratio of the viscous modulus to the elastic modulus, and it measures the existence and degree of elasticity. A gel with a high tan delta tends to have more viscosity than elasticity. By contrast, a gel with a low tan delta tends to have more elasticity than viscosity. The tan delta value of the MHA fillers is higher overall than that of the BHA fillers. Therefore, the biophysical characteristics of MHA fillers result in higher viscosity than elasticity when compared to BHA fillers [16].
After filler application, daily skin changes (e.g., bruises, erythema, edema, aches, tenderness, and itches) were followed up for 14 days. For 15 days after filler application, cases with symptoms (even briefly) and without symptoms were analyzed using the McNemar chi-square test. Among the possible symptoms, bruises and edema were significantly more frequent in the MHA group than in the BHA group. However, no statistical significance was found for other symptoms. In addition, there were five AEs, though none were serious. All AEs were resolved during the clinical trial period. The clinical trial was discontinued in one case due to an AE (partial skin necrosis) at the site of the BHA filler injection. The dropout subject healed without scarring within 2 weeks of follow-up and without special treatment. Importantly, our study demonstrated the safety of administering BHA fillers and the new MHA fillers to NLFs.
Accordingly, we also investigated differences in the biophysical characteristics of the two fillers in terms of efficacy and safety when applied to facial wrinkles such as NLFs. Our study showed that both the BHA filler and the new MHA filler had in vivo safety and efficacy in improving facial wrinkles, and that the new MHA filler was more effective for the cosmetic improvement of wrinkle severity than the BHA filler. Further studies comparing the effects of BHA and the MHA fillers on various types of NLFs are needed to build on the results of the present study, and to provide additional clinically useful data.
In this study, the differences between BHA fillers and the new MHA fillers were analyzed. Meline No. 3 with lidocaine (Bio Standard Inc.), the new MHA filler, and a BHA filler were compared to assess whether there was any difference in their efficacy and safety when correcting NLFs. Consequently, we found that both the BHA filler and the new MHA filler were safe and effective in improving facial wrinkles such as NLFs, and that the new MHA filler was more effective than the BHA filler for the cosmetic improvement of wrinkle severity.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Nowon Eulji Medical Center (IRB No. 2018-10-004) and performed in accordance with the principles of the Declaration of Helsinki.
Patient consent
Written informed consent was obtained from all participants after a full explanation of the risks and benefits of the procedure and the right to determine how personal data is gathered and used, especially when identifiable photographs are used.
Fig. 1.
Flowchart of participants in a comparison study of biphasic hyaluronic acid and monophasic hyaluronic acid fillers. FAS, full analysis/safety set.

Fig. 2.
Photographs of the nasolabial folds (NLFs) of a representative participant before treatment (A), and after filler injection at 24 weeks (B) with the biphasic hyaluronic acid filler (right NLF) and the new monophasic hyaluronic acid filler (left NLF).
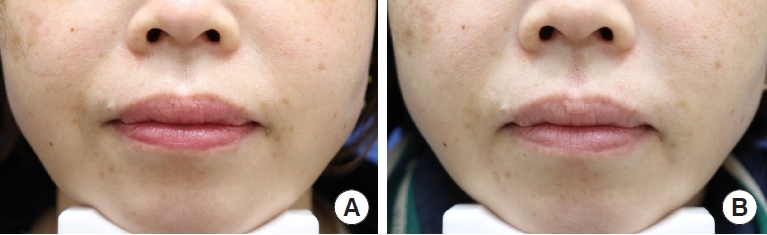
Fig. 3.
Photographs of the nasolabial folds (NLFs) of a representative participant before treatment (A), and after filler injection at 24 weeks (B) with the biphasic hyaluronic acid filler (left NLF) and the new monophasic hyaluronic acid filler (right NLF).
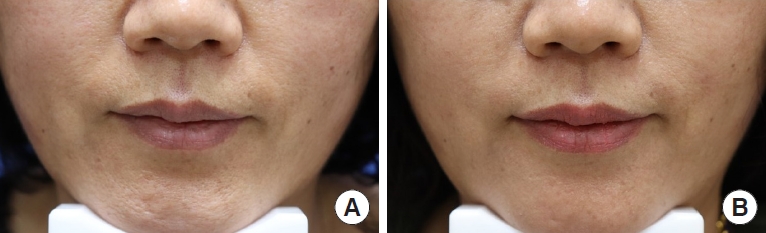
Table 1.
Comparison of the efficacy of BHA and MHA fillers for wrinkle improvement
REFERENCES
1. Necas JB, Bartosikova L, Brauner P, et al. Hyaluronic acid (hyaluronan): a review. Vet Med 2008;53:397-411.

3. Hascell VC, Laurent TC. Hyaluronan: structure and physical properties. Hyaluronan Today 1997;1:A2.
4. Monheit GD. Hyaluronic acid fillers: Hylaform and Captique. Facial Plast Surg Clin North Am 2007;15:77-84.


5. Lapcik L, Lapcik L, De Smedt S, et al. Hyaluronan: preparation, structure, properties, and applications. Chem Rev 1998;98:2663-84.


6. Chung C, Lee JH. A single-center, randomized, double-blind clinical trial to compare the efficacy and safety of a new monophasic hyaluronic acid filler and biphasic filler in correcting nasolabial fold. Aesthetic Plast Surg 2021;45:2902-8.



7. Matarasso SL, Carruthers JD, Jewell ML, et al. Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg 2006;117(3 Suppl): 3S-34S.


8. Hwang E, Song YS. Quantitative correlation between hyaluronic acid filler and hyaluronidase. J Craniofac Surg 2017;28:838-41.


9. Suh JH, Oh CT, Im SI, et al. A multicenter, randomized, double-blind clinical study to evaluate the efficacy and safety of a new monophasic hyaluronic acid filler with lidocaine 0. 3% in the correction of nasolabial fold. J Cosmet Dermatol 2017;16:327-32.
10. Wesley NO, Dover JS. The filler revolution: a six-year retrospective. J Drugs Dermatol 2009;8:903-7.

11. Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg 2011;37:637-43.


12. Gold MH. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interv Aging 2007;2:369-76.




13. Bezzola A, Micheels P. Esthélis®, conception Suisse hyaluronic acid: first comprehensive study of physical-chemical characteristics and clinical trials. Journal de Médecine Esthétique et de Chirurgie Dermatologique 2005;32:11-20.
14. Li X, Xue W, Zhu C, et al. Novel hydrogels based on carboxyl pullulan and collagen crosslinking with 1, 4-butanediol diglycidylether for use as a dermal filler: initial in vitro and in vivo investigations. Mater Sci Eng C Mater Biol Appl 2015;57:189-96.









