|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 29(4); 2023 > Article |
|
Abstract
Background
As rhinoplasty techniques have become more complex, surgeons often need more than what septal or conchal cartilage can provide. While costal cartilage became more popular for that reason, some surgeons are still uncomfortable with its invasiveness and donor site morbidity. Here, we used lyophilized allogeneic costal cartilage for septorhinoplasty and investigated its safety and usefulness.
Methods
The costal cartilage was harvested from a cadaveric donor and treated via multiple steps, including defatting and lyophilization, to remove all viable cells and antigenicity. The cartilage was then stored at room temperature and rehydrated 24 hours before use. Lyophilized cartilage allografts were used in 20 patients. Three types of septal graft were performed â spreader, batten, and extension â to correct septal or columellar deviation and enhance the nasal tip.
Results
The mean follow-up period was 4.3 years. In all cases, the graft successfully met the rhinoplasty purpose. No significant deformation was detected in any of the patients. Although warping was observed in one patient (5%), there was no case of clinical infection, extrusion, or graft removal and no revisional surgery for an unfavorable aesthetic result.
Conclusions
Lyophilized allogeneic cartilage was used for septorhinoplasty very safely and effectively. It can be carved into any shape and has all other properties required for perfectly replacing autologous costal cartilage. The main advantage of cartilage allografts is a limitless supply of high-quality cartilage without donor site morbidity. The disadvantages include the need for special facilities and manpower and extra covering cost.
In conjunction with significant developments in rhinoplasty techniques, demands for high-quality rhinoplasty results have compelled more surgeons to perform complicated septorhinoplasty procedures. Numerous cartilage grafting techniques, such as spreader grafts(whether visible or not), batten grafts, and septal extension grafts, are widely used to enhance the nose shape in primary or secondary rhinoplasty. Accordingly, the need for cartilage grafts has increased. Although autologous cartilage, including the septal or ear cartilage, is considered the best material to meet this demand, the amount of cartilage that can be harvested is often insufficient to meet surgical needs. Moreover, these two types of cartilage might not be available in secondary rhinoplasty. Several recent studies [1-4] have introduced the use of costal cartilage, discussing its utility for aesthetic rhinoplasty, even in female patients. Although all those studies warned of potentially serious problems caused by harvesting costal cartilage, such as a conspicuous scar and a potential risk of pneumothorax, the superior quality and quantity of costal cartilage is garnering increased interest among surgeons.
Costal cartilage allografting is another substitute with comparable quality. Irradiated costal cartilage grafts were first used by Dingman and Grabb in 1961 [5] and multiple clinical reports have been published [6-10]. While some have reported successful uses of costal cartilage homografts for rhinoplasty [11-14], the use of cartilage homografts has not been without controversy, particularly concerning issues of resorption and warping. Some studies [7,15] have claimed that lyophilization can reduce resorption. However, the role of costal cartilage allografts for volume enhancement needs more research to assess its long-term consequences. Therefore, it would be reasonable to limit the use of cartilage allografts only to structural support for the cartilaginous frame of the nose.
We are currently harvesting costal cartilage from cadaveric donors and using it for septorhinoplasty under official approval. The purpose of this study was to investigate the safety and value of the allogeneic lyophilized costal cartilage.
Following the introduction of laws and regulations concerning human tissue and organs by the Korean government in 2005, we established a tissue bank in March 2005 and passed the certification process of the Korean Food and Drug Administration, becoming qualified to harvest, store, and distribute human tissues including bone, cornea, amniotic membrane, vessels, and cartilage. The facilities, equipment, and personnel are of the appropriate quality required by the regulations, and every process of tissue harvest, storage, and distribution is performed strictly according to the standard operating procedure manual. The certification is renewed annually with an on-site audit.
Costal cartilage was procured from cadaveric donors (Fig. 1A) after screening with a transmissible disease blood test. We limited donor age to younger than 40 years old because of age-induced deformation of the cartilage, such as calcification and loss of flexibility. If the harvested cartilage showed any sign of aging, such as yellowish discoloration or loss of shine, the tissue was discarded. After defatting and lyophilization, the harvested cartilage was sealed, sterilized with ethylene oxide, and stored at room temperature. Residual ethylene oxide was checked regularly to maintain the required level. The cartilage was rehydrated in saline and antibiotics for 24 hours before use [16-18].
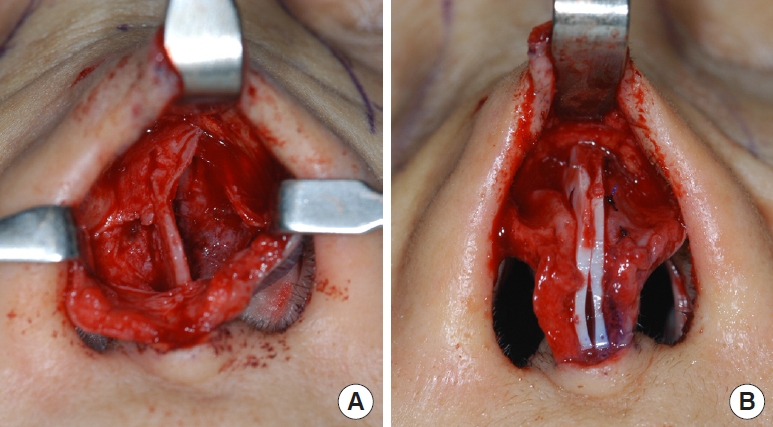
The lyophilized allocartilage block was cut into slices with a thickness of 1â1.5 mm (Fig. 1B). The outermost slices were discarded. The cartilage slices were used in three types of grafts to the septum: spreader grafts, batten grafts, and septal extension grafts. To correct deviations of the dorsal septum, cartilage slices were used as septal spreader grafts. In cases where a saddle deformity was present, cartilage slices were used as visible spreader grafts to smoothen the dorsal septal contour. If the caudal septum was unstable and tended to collapse and deviate, cartilage slices were sutured to one or both sides of the caudal septum as batten grafts to provide structural support (Fig. 2). For patients who wanted to correct a short nose or enhance the nasal tip, slices were used as septal extension grafts (Fig. 3). We retrospectively evaluated outcomes in 20 patients (12 women) who underwent septorhinoplasty with lyophilized allogeneic costal cartilage between December 2007 and January 2011. Because the patients in this study were concerned about scarring of the donor site and wanted a natural change in nasal shape rather than excessive dorsal augmentation, allogeneic lyophilized costal cartilage was used as structural support for the nasal cartilaginous frame. The mean age at operation was 36.2 years (range, 19â63 years). The follow-up period ranged from 2 years to 6.9 years with an mean of 4.3 years. To evaluate postoperative outcomes, preoperative and postoperative photographs of the patientsâ noses were obtained and investigated to identify any relapse of deviation of the dorsal or caudal septal line, saddle deformity, or loss of nasal tip projection.
Among the 20 rhinoplasty cases, 10 patients (50%) had a trauma-related nasal deformity and four patients (20%) had undergone previous surgery, including rhinoplasty or septoplasty. The rest of the patients were candidates for primary septorhinoplasty for aesthetic purposes. The cartilage block recovered to its original condition after rehydration, and the slices were found to be flexible and resilient, but a bit harder than septal cartilage. There were no problems with their use as septal spreader (in 16 cases), batten (in 6 cases), or extension (in 12 cases) grafts. During a follow-up period of at least 2 years, there were no cases of infection or extrusion of the cartilage. No occurrence of infectious disease caused by transmission through blood or body fluid was reported.
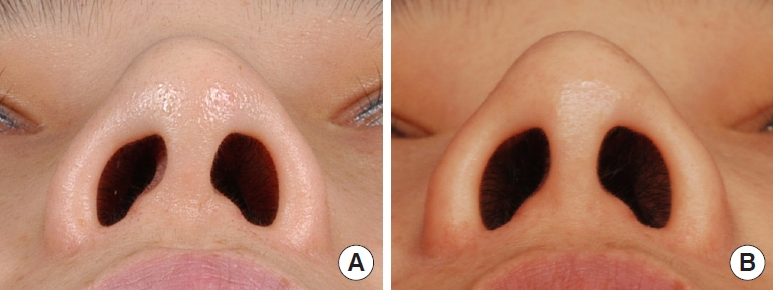
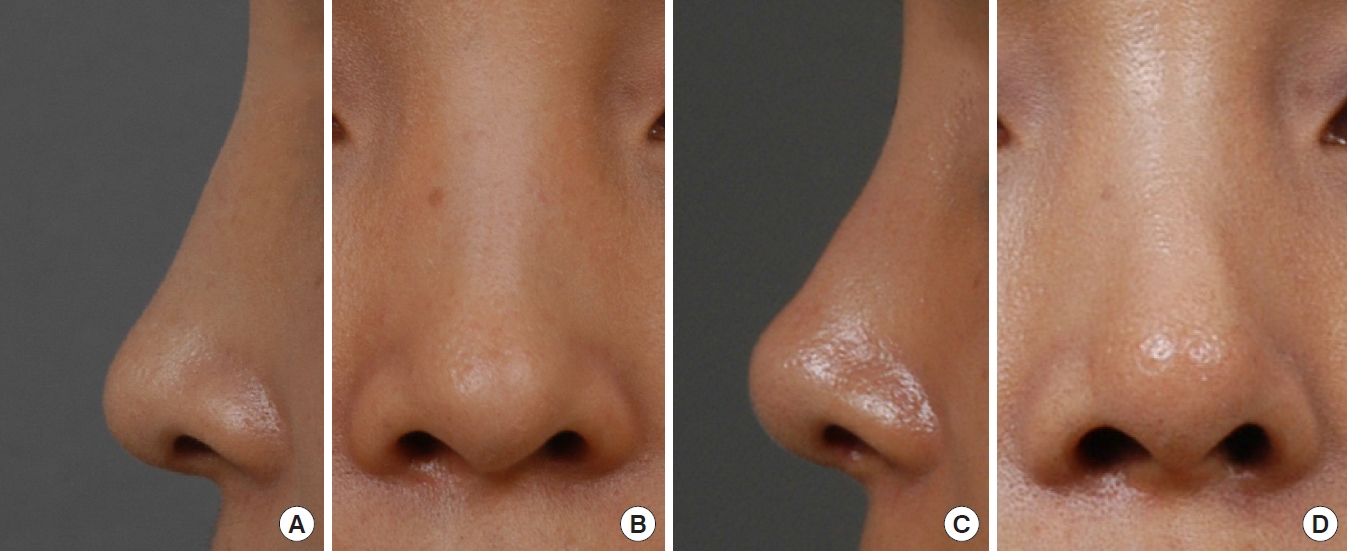
In cases involving spreader grafts, relapse of the dorsal septal deviation or saddle deformity was not found during the follow-up period of 7.9 years. In cases involving caudal septal batten grafts, no relapse of the tip or columellar deviation was noted (Fig. 4) except in one case in which the nasal tip deviated to the right side after 1 year. However, this was a minor complication that did not require reoperation, as the batten graft was primarily performed for structural support in a case where the existing caudal septum was unstable. In cases of septal extension grafts, an apparent improvement could be seen in comparison with preoperative photographs (Fig. 5), although cephalic rotation and loss of projection of the nasal tip were noted over time. However, there was no case in which the change was so serious that revisional surgery was necessary.
Allogeneic costal cartilage has been reported to be a very reliable and versatile graft material for rhinoplasty [6,10-13]. However, it has been also claimed that most of the volume of an allocartilage graft will eventually be lost [18-20], and we did not expect that all the lyophilized cartilage grafts would maintain their volume permanently. Thus, we used lyophilized allocartilage for spreader, batten, and extension grafts to support the pre-existing framework. Even if the allocartilage is gradually resorbed with time, the corrected shape and position of the septum or lower lateral cartilage will be maintained along with the fibrotic change of the surrounding soft tissue. For septal extension grafts, however, the additional increase in the length of the septum can be partly lost with time because anterior and caudal extension was only made by the allocartilage.
As a cartilage processing method, lyophilization has the advantages of long-term storage and easy handling [21]. It enables the long-term storage of cartilage at room temperature. Lyophilization is also helpful for eradicating microorganisms that can cause fatal complications. Furthermore, the lyophilization process greatly reduces the antigenicity and electric potential of the cartilage, thus reducing tissue reactions after grafting and the resorption rate [7,15]. Though the lyophilization process makes the cartilage block stiff, rehydration for 24 hours revives the basic characteristics of the cartilage as an excellent biomaterial. Lyophilized allogeneic cartilage can be carved into any shape and has all other properties necessary to replace autogenous costal cartilage. We successfully applied the cartilage grafts to correct septal deviations or enhance the nasal tip definition and projection without concern about material unavailability and donor morbidity. Although our study was limited by the small number of patients, our results showed the usefulness and stability of lyophilized cartilage.
Lyophilized cartilage can be sterilized either by radiation or ethylene oxide. Inconsistent results have been reported about the effect of radiation on the resorption rate. While a number of studies have reported successful grafts of radiated cartilage with the least resorption [9,10,22,23], there have been studies reporting higher and earlier resorption of the radiated cartilage. Donald [19] reported in a long-term study of up to 72 months that complete resorption of irradiated cartilage was observed in 87.7% of cases versus 43.8% of cases of chemical-stored cartilage. Wangerin et al. [24] reported that radiation-sterilized lyophilized cartilage was completely resorbed after 125 days, while ethylene oxide-sterilized lyophilized cartilage was partially resorbed after 328 days and maintained a constant form. Recently, Lee et al. [21] reported no difference in the resorption rate between radiation-sterilized cartilage and ethylene oxide-sterilized cartilage. However, the latter study used heterogenic cartilage and might have been affected by remaining antigenicity. In our present study, we used lyophilized cartilage without radiation, because we concluded that lyophilization is beneficial and radiation can potentially cause an adverse effect. The use of lyophilized costal cartilage allograft without radiation can cause concerns about safety issues such as inappropriate sterilization. In our case series, however, there were no infection-related complications, which supports the safety of this method.
The most distinct advantage of using lyophilized allocartilage over autologous cartilage is a limitless supply of high-quality cartilage without donor site morbidity. As we could harvest almost 20 blocks of costal cartilage from a single cadaveric donor, there was no problem with availability. As long as cadaveric organ transplantation continues, we can secure the supply of cartilage. However, opportunities to procure cartilage from cadavers can be sporadic because the distribution of cadaveric donors among hospitals is strictly controlled by the government. One of the drawbacks of using lyophilized allocartilage grafts is the need for special facilities, equipment, and experienced personnel during the process of harvest, treatment, and storage. Another drawback of allocartilage is its additional cost, which is equivalent to 500 US dollars.
The limitations of this study include the small number of cases, but this study makes a substantial contribution by showing that septal spreader (in 16 cases), batten (in 6 cases), or extension (in 12 cases) grafts can be utilized and confirming their usefulness and stability over a relatively long follow-up period (mean, 4.3 years; range, 2â6.9 years), with no cases requiring infection or reoperation. We are confident that our results can be confirmed in future studies with a larger number of cases, and that this study can serve as a springboard for the continued exploration of cartilage alternatives that can be utilized for high-quality rhinoplasty.
In conclusion, lyophilized costal cartilage could be used as an alternative graft material in septorhinoplasty when autologous septal cartilage is either insufficient or unsuitable. The continued evaluation of these cases will provide even more valuable information pertaining to the longevity of lyophilized costal cartilage allografts.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Asan Medical Center (IRB No. S2023-0170-0001) and performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Patient consent
The patients provided written informed consent for the publication and use of their images.
Fig. 1.
(A) Blocks of harvested costal cartilage from a cadaveric donor. (B) Slices of allogeneic cartilage that were divided from the hydrated cartilage block. They are as soft and flexible as the harvested autologous cartilage.

Fig. 2.
(A) Isolated caudal septum is found to be deviated and collapsed, which manifested as columellar deviation. (B) Septal batten graft using allogeneic costal cartilage slice was fixed to the left side of the caudal septum to provide support. (C) Batten grafts were applied to both sides of the caudal septum.
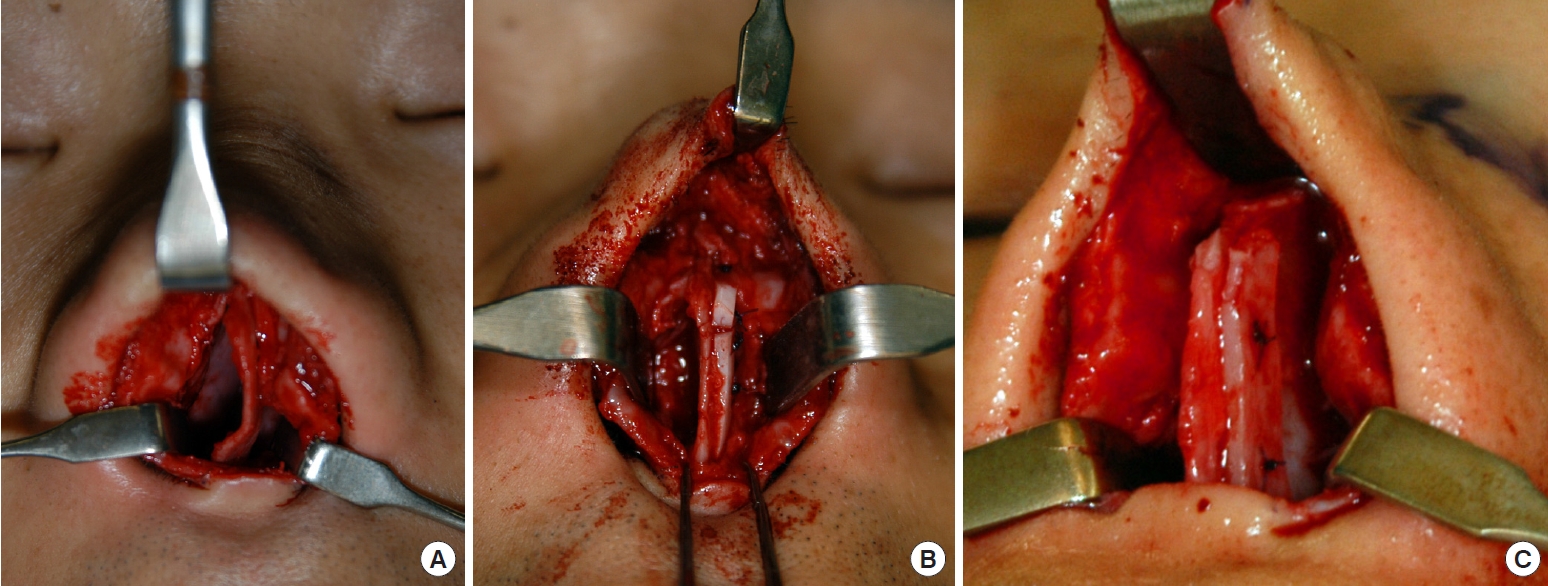
Fig. 3.
(A) The caudal septum was exposed and found to be thin enough to be easily deflected. (B) Two slices of allogeneic cartilage were placed at both sides of the caudal septum for septal reinforcement and extension to dorsal and caudal direction.
REFERENCES
1. Sajjadian A, Rubinstein R, Naghshineh N. Current status of grafts and implants in rhinoplasty: part I. Autologous grafts. Plast Reconstr Surg 2010;125:40e-9e.
2. Yilmaz M, Vayvada H, Menderes A, et al. Dorsal nasal augmentation with rib cartilage graft: long-term results and patient satisfaction. J Craniofac Surg 2007;18:1457-62.

3. Cervelli V, Bottini DJ, Gentile P, et al. Reconstruction of the nasal dorsum with autologous rib cartilage. Ann Plast Surg 2006;56:256-62.


4. Marin VP, Landecker A, Gunter JP. Harvesting rib cartilage grafts for secondary rhinoplasty. Plast Reconstr Surg 2008;121:1442-8.


5. Dingman RO, Grabb WC. Costal cartilage homografts preserved by irradiation. Plast Reconstr Surg Transplant Bull 1961;28:562-7.


6. Muhlbauer WD, Schmidt-Tintemann U, Glaser M. Long-term behaviour of preserved homologous rib cartilage in the correction of saddle nose deformity. Br J Plast Surg 1971;24:325-33.


7. Sailer HF. Experiences with the use of lyophilized bank cartilage for facial contour correction. J Maxillofac Surg 1976;4:149-57.


8. Sailer HF, Gratz KW, Kalavrezos ND. Frontal sinus fractures: principles of treatment and long-term results after sinus obliteration with the use of lyophilized cartilage. J Craniomaxillofac Surg 1998;26:235-42.


9. Strauch B, Wallach SG. Reconstruction with irradiated homograft costal cartilage. Plast Reconstr Surg 2003;111:2405-13.


10. Burke AJ, Wang TD, Cook TA. Irradiated homograft rib cartilage in facial reconstruction. Arch Facial Plast Surg 2004;6:334-41.


11. Lefkovits G. Irradiated homologous costal cartilage for augmentation rhinoplasty. Ann Plast Surg 1990;25:317-27.


12. Kridel RW, Ashoori F, Liu ES, et al. Long-term use and follow-up of irradiated homologous costal cartilage grafts in the nose. Arch Facial Plast Surg 2009;11:378-94.


13. Demirkan F, Arslan E, Unal S, et al. Irradiated homologous costal cartilage: versatile grafting material for rhinoplasty. Aesthetic Plast Surg 2003;27:213-20.



14. Menger DJ, Nolst Trenite GJ. Irradiated homologous rib grafts in nasal reconstruction. Arch Facial Plast Surg 2010;12:114-8.


15. Pate JW, Sawyer PN. Freeze-dried aortic grafts; a preliminary report of experimental evaluation. Am J Surg 1953;86:3-13.

16. Obwegeser HL. Correction of the skeletal anomalies of oto-mandibular dysostosis. J Maxillofac Surg 1974;2:73-92.


17. Komender J, Marczynski W, Tylman D, et al. Preserved tissue allografts in reconstructive surgery. Cell Tissue Bank 2001;2:103-12.

18. Kim EK, Lee TJ. Use of lyophilized allogeneic costal cartilage: is it effective to maintain the projection of the reconstructed nipple? Ann Plast Surg 2011;66:128-30.

19. Donald PJ. Cartilage grafting in facial reconstruction with special consideration of irradiated grafts. Laryngoscope 1986;96:786-807.


20. Sajjadian A, Naghshineh N, Rubinstein R. Current status of grafts and implants in rhinoplasty: part II. Homologous grafts and allogenic implants. Plast Reconstr Surg 2010;125:99e-109e.
21. Lee JH, Chang CH, Seo SW, et al. The effects of sterilization methods on lyophilized cartilage grafts in an experimental model. J Craniofac Surg 2013;24:1436-40.


22. Donald PJ, Wildes TO, Miller DC, et al. Radiologic evaluation of irradiated cartilage grafts on the facial skeleton of sheep. Head Neck Surg 1980;2:483-6.


-
METRICS

-
- 0 Crossref
- 921 View
- 102 Download
- Related articles in AAPS
-
Editorial: Septal Cartilage Graft for Nasal Tip.1999 March;5(1)
Rib/costal Cartilage Combination Graft in Rhinoplasty.2005 September;11(2)
10th Rib Cartilage: Another Option of the Costal Cartilage Graft for Rhinoplasty2015 June;21(2)