Efficacy of ultrasonic cavitation in isolation of the stromal vascular fraction from adipose tissue
Article information
Abstract
Background
Adipose tissue-derived stem cells (ASCs) represent a rapidly evolving area of cell-based therapies. ASCs are harvested from the stromal vascular fraction (SVF), a heterogeneous mixture of ASCs and other cell types. Two methods are typically employed to obtain the SVF: enzymatic and mechanical. In this study, we examined the efficacy of ultrasonic cavitation in isolating the SVF from adipose tissue.
Methods
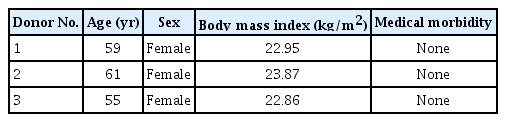
Human adipose tissue was procured from three patients through an aesthetic liposuction procedure. This tissue was then subjected to either an enzymatic method utilizing type II collagenase or an ultrasonic cavitation method using an Ultra Stemcell device. The cell counts and viability were determined using a cell counter. The immunophenotype of the SVF was analyzed using real-time polymerase chain reaction.
Results
Compared to the enzymatic method, the total cell count and cell viability of the SVF isolated through ultrasonic cavitation were relatively low. However, no significant difference was observed in the immunophenotype of CD45, CD11b, CD34, and CD105.
Conclusions
The ultrasonic cavitation method constitutes a clinically practical approach that offers straightforward access in the operative field. This technique is a rapid and cost-effective method for isolating the SVF.
INTRODUCTION
Adipose tissue-derived stem cells (ASCs) are rapidly gaining prominence in the field of cell-based therapy due to their multipotent capacity to differentiate into various cell types, including adipocytes, osteoblasts, myoblasts, chondrocytes, smooth muscle cells, endothelial cells, neurons, and hepatocytes [1]. The stromal vascular fraction (SVF) is a heterogeneous mixture of cells that includes ASCs, along with other cell types such as endothelial cells, erythrocytes, and immune cells. The SVF has demonstrated effectiveness in treating a range of diseases, including scleroderma, radiotherapy-induced tissue damage, peripheral neuropathy, osteoarthritis, and diabetic foot ulcers [2]. Consequently, the need for a suitable extraction method for the various clinical applications of SVF in the medical field is becoming increasingly apparent. Two primary methods are employed to obtain the SVF: enzymatic and mechanical. Enzymatic processes are among the most commonly used methods for SVF isolation. However, these enzymatic methods come with certain drawbacks. The first is the safety concern associated with tissue dissociation enzyme mixtures [3]. The second is the substantial cost involved when producing the SVF using collagenase [4]. The purpose of the present study was to introduce a mechanical method and evaluate its efficacy in isolating the SVF from lipoaspirates via ultrasonic cavitation.
METHODS
Preparation of fat for SVF isolation
Adipose tissue was obtained from the abdominal region of patients who were undergoing elective cosmetic liposuction under general anesthesia. The liposuction was manually performed using a blunt cannula and a 50-cc syringe, applying 30 cc of negative pressure. Three patients underwent the procedure, with 60 cc of lipoaspirate collected from each patient (Table 1). Individuals with a history of metabolic diseases or steroid or immunosuppressant injections were excluded from the study. Half of the adipose tissue harvested from each patient was subjected to an enzymatic method, while the remaining half was processed using a mechanical approach, specifically ultrasonic cavitation.
Preparation of dermal fibroblasts for the control group
Full-thickness skin was procured from the same patient for the extraction of dermal fibroblasts. The harvested skin underwent a process of defatting, along with the removal of the epidermis. The dermis was then subjected to a washing step using Hank’s balanced salt solution and subsequently incubated at 37 °C for 90 minutes with collagenase type I (Sigma-Aldrich). Following this, 10% fetal calf serum was introduced to inactivate the collagenase. The resulting cell suspension was filtered using a 100-μm cell strainer. Next, the suspension was centrifuged at a speed of 1,200 rpm for 5 minutes. Finally, dermal fibroblasts were obtained by resuspending the cells in Dulbecco’s modified Eagle medium (DMEM).
Isolation of the SVF via enzymatic method
After being washed five times with Hank’s balanced salt solution and a 2% solution of bovine serum albumin, the adipose tissue samples were sectioned into small pieces. These tissue samples were subsequently incubated at 37 °C for 30 minutes in collagenase type II (Sigma-Aldrich). The resulting suspension was washed with DMEM (Sigma-Aldrich) that was supplemented with 10% fetal bovine serum (Invitrogen Corp.), 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific), and 0.2% amphotericin B (Fungizone; Sigma-Aldrich) to inactivate the collagenase. Following digestion, the resulting suspension was filtered through a sterile 100-μm cell strainer to remove any undigested parts. The remaining suspension was then centrifuged at 2,000 rpm for 10 minutes to yield a high-density pellet composed of the SVF. Following centrifugation, the lipid layer was removed, and the SVF was collected. To lyse the erythrocytes, red blood cell (RBC) lysis buffer was utilized. The mixture was then recentrifuged at 2,500 rpm for 10 minutes. The supernatant was discarded, and the SVF pellet was resuspended in phosphate-buffered saline. This procedure was repeated until a white pellet was obtained.
Isolation of the SVF via ultrasonic cavitation
The processed adipose tissue was placed into a syringe and allowed to undergo gravity sedimentation for 30 minutes. The resulting suspension was filtered using a sterile 100-μm cell strainer. The tissue was subsequently gathered in a bottle and subjected to ultrasonication with an Ultra Stemcell device (Medifutures) for 7 minutes at 25 MHz to achieve homogenization. This process disrupted the adipocytes. The sample was then transferred to a conical tube and centrifuged at 3,000 rpm for 2 minutes. Following centrifugation, the lipid layer was discarded, and RBC lysis buffer was added to eliminate erythrocytes. The suspension was then centrifuged again at 3,000 rpm for 10 minutes. The supernatant was discarded, and the SVF pellet was resuspended in phosphate-buffered saline. This procedure was repeated until a white pellet was obtained.
In vitro evaluation of SVF cell count and viability
Cell counts and viability were assessed using an automatic cell counter (ADAM-MC; NanoEntek), in accordance with the manufacturer’s guidelines. Cell suspensions derived from each processing method were seeded onto plates. Subsequently, two types of AccuStain solutions were introduced to stain the total cell population and the non-viable cells. After the total and non-viable cells were counted, the viability was calculated.
In vitro evaluation of SVF immunophenotype
To confirm whether the two SVF isolation procedures altered the SVF composition, the expression of SVF cell surface markers was analyzed using real-time polymerase chain reaction (PCR). Isolated RNA was converted into complementary DNA (cDNA) using an Easy cDNA Synthesis Kit (NANOHELIX). The isolated RNA template was combined with random hexamers and RNase-free water. The samples were then incubated at 65 °C for 5 minutes and immediately placed on ice. Then, 4 μL of 5× transcriptase buffer and 1 μL of reverse transcriptase were added. Next, the sample was incubated at 50 °C for 50 minutes and then at 42 °C for 10 minutes, both times with gene-specific primers. Finally, the cells were incubated at 70 °C for 10 minutes to halt the reactivation. The synthesized cDNA was subsequently used for PCR.
The PCR amplification of SVF cell markers, namely CD45, CD105, CD11b, and CD34, was performed in a reaction mixture containing 2 μL of DNA template, 10 μL of SYBR green dye, 6 μL of RNase-free water, and 1 μL each of forward and reverse primers. The thermal cycling conditions were set to include an initial denaturation period at 95 °C for 5 minutes. This was followed by 40 cycles, each consisting of 20 seconds at 95 °C, 30 seconds at 58 °C, and 30 seconds at 72 °C. The PCR amplification reaction of identical human dermal fibroblasts served as the control group for comparison. The products of the PCR amplification were visualized using a real-time PCR system (StepOne; Thermo Fisher Scientific).
Statistical analysis
Statistical analyses were performed using SPSS software (version 26; IBM Corp.). The total cell count and viability of SVF, obtained through both isolation methods, were analyzed using the Mann-Whitney U test.
RESULTS
Effects of both isolating methods on cell count and viability
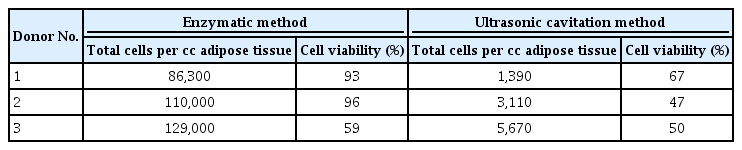
Table 2 and Fig. 1 illustrate the results for each donor’s adipose tissue. The total number of SVF cells harvested from 1 cc of adipose tissue was 108,000±21,000 using the enzymatic method and 3,000±2,000 using the ultrasonic cavitation method (P=0.100). The viability of the SVF cells was 82.67%±20.55% in the enzymatic group and 54.67%±10.79% in the ultrasonic cavitation group (P=0.200) (Table 3).
Cell counts and viability of stromal vascular fraction obtained using enzymatic and ultrasonic cavitation methods
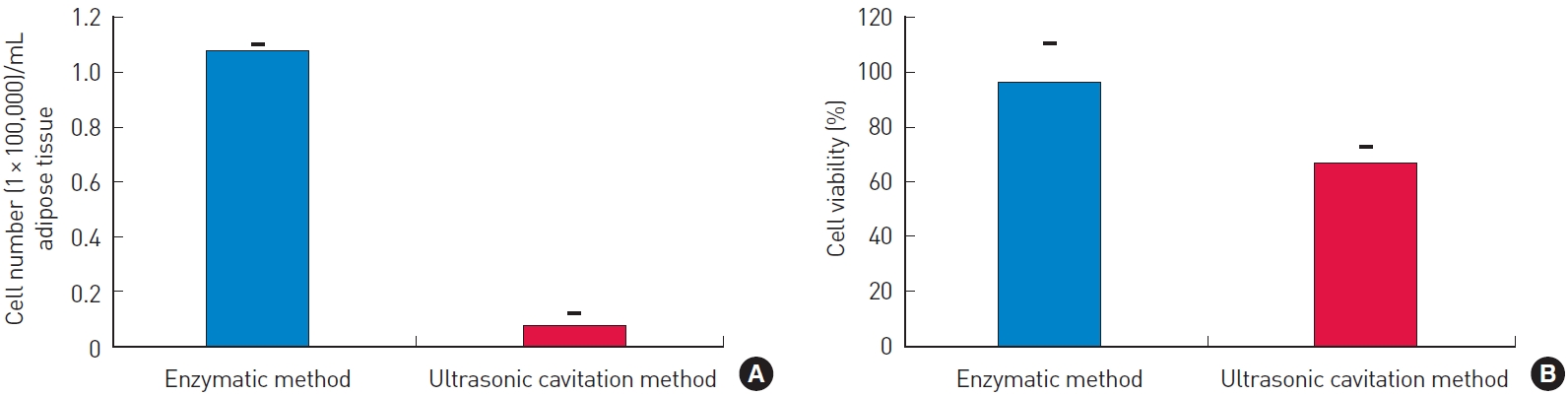
Cell count and viability of stromal vascular fraction (SVF) obtained using enzymatic and ultrasound-mediated mechanical methods. (A) The total cell count of SVF obtained from the ultrasonic cavitation method was significantly lower than that of SVF from the enzymatic method. (B) There was no significant difference in cell viability of SVF from each method.
Evaluation of immunophenotype of SVF cells
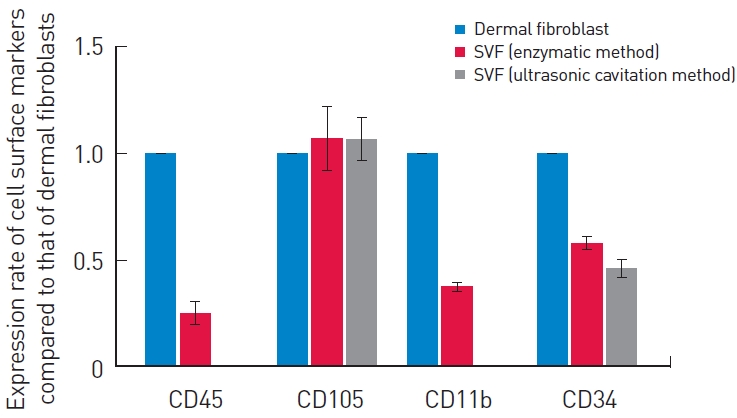
After each isolation method was applied, the immunophenotype of the SVF was assessed. The expression of a subset of cell surface markers in the prepared SVF was analyzed using real-time PCR (Fig. 2). SVF cells from both isolation methods exhibited similar cell surface markers, with positive expression of CD105 and CD34 markers and negative expression of CD45 and CD11b markers, in comparison to dermal fibroblasts. The SVF derived from ultrasonic cavitation demonstrated a relatively lower expression of CD45, CD11b, and CD34, and a higher expression of CD105, compared to the enzymatic process.
DISCUSSION
Two primary techniques are commonly employed to extract the SVF from adipose tissues: enzymatic and mechanical methods. The enzymatic digestion of adipose tissue is the most frequently used method for SVF isolation. In brief, the adipose tissue undergoes a washing process, followed by enzymatic digestion. The cells are then separated from the mature adipocytes, released oil, and enzyme solution through centrifugation. Prior research has demonstrated that the enzymatic dissociation of fat tissue facilitates the extraction of viable and proliferative ASCs [1,5,6]. However, the use of enzymes raises safety concerns [7-10]. Chang et al. [10] reported that residual collagenase in ASC resulted in some variations in biochemical analyses and organ weights, but it did not significantly impact clinical signs, mortality, or body weight in mice. However, few studies are available on the safety of collagenase in humans. Furthermore, since the enzymes used for SVF cultivation are derived from animals, safety issues related to these animal-derived reagents are inevitable. Several complications associated with animal-derived reagents have been reported, including severe anaphylaxis, immune reactions, and viral and bacterial infections [11,12].
Thus, the creation of non-enzymatic methods could address these problems. Numerous mechanical strategies exist for isolating the SVF, including washing steps, vibration and shaking procedures, centrifugation through the application of shearing force, and pressure [4,13-15].
Mechanical methods have several distinct advantages. Enzymatic methods necessitate an extended isolation period for the digestion of adipose tissue to separate the cellular components; additionally, these techniques are relatively costly. In contrast, the mechanical method can disrupt adipocytes to procure SVF swiftly and inexpensively [15,16]. The total processing duration for the enzymatic method is approximately 2–3 hours, whereas the mechanical method typically takes less than an hour [13,14,17].
Despite its advantages, the mechanical method has a drawback: it yields fewer cells than the enzymatic method [13]. The total cell count obtained from 1 cc of adipose tissue using the mechanical and enzymatic methods varied between 10,000–140,000 and 5,000–7,100,000, respectively. However, when comparing the mechanical method with the enzymatic approach, the viability of the SVF cells was comparably preserved. Furthermore, previous studies that employed mechanical methods, such as vortexing and centrifugation, the intersyringe process, and repeated washing, have indicated that the extracted SVF demonstrated similar adipogenic and osteogenic differentiation [4,14].
A mechanical approach that employs ultrasound is a valid technique for confirming the differentiation potential of ASCs. As reported by Amirkhani et al. [17], SVF isolated through ultrasonic cavitation exhibited similar surface markers when analyzed by flow cytometry. No significant differences were found in the differentiation potential of ASCs isolated using ultrasonic cavitation and the enzymatic method. In this study, we assessed a novel technique that utilizes ultrasound-mediated cavitation to homogenize fat tissue and partially disrupt adipocytes for SVF extraction. The shearing forces of cavitation disrupt the adipose tissue, liberating stem cells from the fat tissue structure.
In general, ultrasound-based methods of SVF isolation yield significantly fewer cells compared to enzymatic methods [18]. In our study, the ultrasound device produced an average cell yield of 3,390, with a cell viability of 54.67%. This was lower than the enzymatic method, which yielded 108,430 cells and a viability of 82.67%.
We hypothesize that several factors contribute to these observed discrepancies. First, the difference in yields between the mechanical and enzymatic methods can be partially attributed to the physical location of SVF cells within the adipose tissue. SVF cells, particularly mesenchymal stem cells and pericytes, tend to be localized in the perivascular space [19]. Mechanical methods lack the capacity to destroy extracellular matrix in a similar fashion to enzymatic methods. Therefore, many cells that were not separated from their surrounding tissues were removed during the filtering process. Second, the ultrasound frequency may have impacted the cellular structure. According to Fraser et al. [18], high ultrasound energy can cause a significant increase in tissue temperature and disrupt cellular structures, leading to cell death. This could have contributed to the reduced yield and viability of the SVF.
To identify the potential of stem cells within the SVF, it is necessary to employ a surface marker to distinguish the cellular components. However, the SVF is a heterogeneous cellular mixture, comprising hematopoietic cells, endothelial cells, pericytes, and stromal cells. Consequently, no single marker can be used to identify the SVF [6]. To discern the SVF constituents, it is necessary to detect multiple markers, utilizing combinations of both negative and positive markers. According to the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy, the immunophenotype of SVF is positive for CD13, CD29, CD34, CD44, CD73, and CD90 and negative for CD31 and CD45. Additionally, four other markers, namely CD13 (APN), CD73 (L-VAP-2), CD90 (Thy-1), and CD105 (Endoglin), have been employed to identify the SVF [5]. From the various markers, we selected four—CD45, CD105, CD11b, and CD34—for use in SVF identification. In essence, we aimed to ascertain the capabilities of both immune cells and stem cells by using these four surface markers. The relative quantification of cell surface markers of the SVF, obtained through both enzymatic and mechanical methods, was performed using real-time PCR. Consequently, we were able to confirm the capacity of stem cells in the SVF obtained via ultrasonic cavitation. In this study, we examined the cellular components of the SVF using surface markers to determine the composition and proportion between the two methods. The cells isolated using our method exhibited positive expression of CD34 and CD105 and negative expression of CD45 and CD11b, aligning with the immunophenotype of the SVF.
The differences in the immunophenotype of the SVF between enzymatic and mechanical methods can be attributed to the fact that enzymatic digestion breaks down collagen in the extracellular matrix, thereby releasing a greater number of adipose-derived stem cells. In contrast, the mechanical method extracts only a subpopulation of these stem cells [4]. The various isolation methods result in differing proportions of cell types, which are subsequently responsible for the observed differences in immunophenotype.
Dermal fibroblasts were utilized as the control group for relative quantification via real-time PCR for two reasons. First, dermal fibroblasts, which are a heterogeneous population consisting of progenitors with varying levels of differentiation potential, display a surface immunophenotype resembling that of mesenchymal stem cells. They are also clinically used for the treatment of nonhealing wounds due to their mesenchymal properties [20,21]. As such, we sought to validate the stem cell properties of the SVF by comparing it to dermal fibroblasts. Second, the relative ease of procuring dermal fibroblasts compared to mesenchymal stem cell provides an advantage. These cells can be readily collected in the operative field, making them particularly convenient for use in experimental studies.
Our findings suggest that alternative ultrasonic cavitation methods can be useful for SVF isolation. Although the yield and viability of SVF cells were lower than those obtained through the enzymatic method, these cells retained their stem cell characteristics, as confirmed by real-time PCR. The processing time for our technique was 15 minutes, compared with 3 hours for the enzymatic method. Furthermore, this ultrasonic cavitation approach is cost-effective, particularly considering the high cost of the enzymatic method ($210 per disposable unit for the ultrasonic cavitation method vs. $460–$1,950 per disposable unit for the enzymatic method) [19].
This study does present several limitations. First, the sample size was insufficient to evaluate the efficacy of both the enzymatic and mechanical isolation methods. Second, the immunophenotype of SVF was identified solely through relative quantification using real-time PCR. Third, we did not evaluate the differentiation capacity of SVF using either isolation method. Consequently, additional in vivo and in vitro studies have been planned to incorporate a larger patient population and diverse patient groups. Furthermore, the immunophenotype will be confirmed by employing additional surface markers and fluorescence-activated cell sorting to determine absolute values.
In conclusion, our ultrasonic cavitation method resulted in a low cell count and viability of the harvested SVF, which aligns with previous findings. However, we successfully confirmed the immunophenotype through additional real-time PCR analysis, thereby validating its stem cell properties. Considering the potential for clinical application, the suitability of this method in the surgical field, coupled with the time and cost implications of each approach, suggests its potential clinical efficacy.
Notes
Eun Soo Park is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Ethical approval
The study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (IRB No. 2021-03-003-008) and performed in accordance with the principles of the Declaration of Helsinki. All participants provided informed consent.