 |
 |
- Search
| Arch Aesthetic Plast Surg > Volume 21(2); 2015 > Article |
Abstract
Background
Medium-sized congenital melanocytic nevi (CMN) require surgical excision because of the risk of malignant transformation and aesthetic concerns. There are various possible reconstruction methods after excision, such as primary repair, skin graft, local flap, and composite graft. In this study, we used skin-fat composite grafts for reconstructing full-thickness skin defects and assessed the aesthetic outcomes.
Methods
Facial nevi excision plus skin-fat composite grafts were performed in 11 children (range, 3-16 years old). All grafts were harvested from the preauricular area on one or both sides; they included the epidermis, full-thickness dermis, and subcutaneous fat. All procedures were performed simultaneously. Standardized photographs were taken preoperatively and at a mean follow-up of 10.5 months. Viewing the photographs, four plastic surgeons rated the aesthetic outcomes of all patients using the following scale: 1, poor; 2, fair; 3, good; 4, very good; and 5, excellent.
Results
No patient was diagnosed with malignancy. There were no complications, such as graft loss, infection, or aesthetic problems that required surgical revision. All donor sites healed well and exhibited only minimal scarring. The aesthetic outcomes of skin-fat composite grafts were outstanding with a mean score of 4.2 +/- 0.4. Most patients and parents were highly satisfied with the results.
Conclusions
Skin-fat composite grafts provide good color match, texture, and contour. They are more tolerable, especially in children, because the technique involves simpler procedures and shorter operation times than local flaps. They may be the ideal option for facial defects after excision of medium-sized melanocytic nevi.
Congenital melanocytic nevi (CMN) are defined as melanocytic nevi that present at birth or within the first few months of life. Small or medium-sized nevi occur in approximately 1% to 6% of all newborns, whereas giant nevi occur in approximately 1 in 20,000 [1-3].
There are two main considerations in the treatment of CMN. The first is predicting the risk of its transformation to melanoma, and the second is aesthetic satisfaction. Generally, it is known that the bigger the nevus, the greater the risk of malignant transformation. The risk of melanoma transformation of small and mediumsized CMN is controversial, but it is generally thought to be less than 4.9% [4]. Currently, there is no consensus regarding the management of small and medium-sized CMN, whereas interventions are highly recommended early in life for giant congenital nevi. Nevertheless, even a small nevus on the face or other visible area may precipitate the need for surgery for cosmetic and psychological reasons. Some surgeons believe that surgical management should be considered early, so that excision of the nevus and scar maturation can be completed before they have a psychological impact on the child [5].
The surgical and reconstructive options for CMN differ depending on the size and location of the facial lesion. Although some authors have reported the use of lasers for treating nevi, this is unlikely to be successful because the deeper portions of CMN are not affected by laser therapy [5,6]. Dermabrasion may be another option, but if the treatment depth is too superficial, the risk of recurrence rises, and if the treatment depth is too deep, substantial scarring results and negates the aesthetic purpose [5]. Thus, the main approach to treatment of facial CMN is surgical excision, and the best option for a small nevus is excision and primary closure. However, this is inappropriate for CMN that are too large to be closed primarily or for nevi located close to the eyelids, nose, or mouth, as it will distort the adjacent tissues. In these cases, the surgeon must meticulously try to predict the aesthetic benefit when planning the surgical procedure.
There are several surgical options for CMN, including partial thickness excision, serial excision, excision with skin graft, and a local flap. Partial thickness excision combined with a split-thickness skin graft (STSG) has the potential disadvantages of not only the potential for recurrence but also substantial deformities related to color match and contours. Although these techniques have a role in the management of CMN of the extremities, their poor aesthetic outcomes can be a major drawback for facial CMN. Serial excision enables full thickness excision of the lesion and wound closure with a shorter scar [6]. However, this technique requires multiple surgical procedures and may create tissue distortion, which makes it difficult to be used for CMN near sensitive areas of the face. Local flaps are generally preferred over full-thickness skin grafts (FTSGs) and skin-fat composite grafts in reconstructing facial skin defects. A local flap using adjacent tissues provides better color match and texture than skin grafts, as well as sufficient volume. However, a local flap requires a more complex surgical technique, and it may increase contour deformities of the surrounding tissues, scarring due to additional incisions, and dog-ears, all of which may occasionally necessitate additional reparative surgery [7]. Creation of a FTSG is a simpler procedure than a local flap, and it results in better skin color and texture than STSGs. Yet, the possibility of color mismatch and contour deformities due to volume depletion are drawbacks of FTSGs. As another option, a skin-fat composite graft including the epidermis, full-thickness dermis, and subcutaneous fatis relatively free from the complications of a local flap or the possibility of volume depletion associated with a FTSG, because the fat component of the graft can provide a better structural frame and graft volume than a FTSG. Still, a skin-fat composite graft has the potential drawbacks of color mismatch and contour deformity.
In this study, we discuss the advantages and cosmetic benefits of a skin-fat composite graft as a reconstructive method of full-thickness skin defects after excision of medium-sized CMN in children.
We performed a retrospective study of 11 patients with facial CMN, who underwent total excision of the nevus plus a skin-fat composite graft from November 2004 to March 2012 at a single institution. The patients included 4 males and 7 females, who were 3 to 16 years old (mean, 7.6 years old). Three CMN involved the upper or lower eyelids, three involved the oral commissure or lips, and five involved the nose or cheek adjacent to the ala. Nevus excision and skin-fat composite grafting were performed in a one-stage operation. The mean follow-up period was 13.2 months, ranging from 6 to 32 months (Table 1).
All operations were performed under general anesthesia. All patients underwent complete excision of their facial nevus. For each skin defect, we made a paper template based on the skin defect and designed an elliptical-shaped graft including the template in the preauricular area. Pinching the donor site skin, we made the decision to use grafts from the preauricular area on one or both sides of the face, based on whether the donor site could be closed primarily. The graft harvesting included full-thickness skin and subcutaneous fat, and each donor site underwent primary closure. After placing the harvested graft at the defect, we partly defatted and trimmed the graft using curved scissors, depending on the shape and depth of the skin defect. The graft was then sutured into the defect with 6-0 black silk.
Standardized photographs were taken preoperatively, immediately after surgery, and after a mean follow-up period of 10.5 months. The serial photographs of all patients were clinically evaluated by four plastic surgeons who were not involved with the operations. The evaluated aesthetic aspects included skin color, texture, and contour. They were assessed using the following grading scale: 1, poor; 2, fair; 3, good; 4, very good; and 5, excellent.
Pathologically, six patients had compound nevi, four had intradermal nevi, and only one had both a compound and intradermal nevus. No patient was diagnosed with a malignancy. The mean size of the skin-fat composite grafts was 1.9×1.3 cm2, with a range of 1.0×0.5 to 2.5×2.5 cm2. The size and shape of each graft was appropriate to the skin defect. For nine patients who underwent the one-stage operation, either the left or right preauricular area was used as the donor site. For two patients with larger nevi, both sides were used to obtain two or three skin-fat composite grafts, based on the defect size and aesthetic units (case 1, case 5).
No complications, such as recurrence, infection, contracture of adjacent tissues, or hypertrophic scar at the graft boundaries, developed and only one patient required additional surgery for remnant nevus. Every donor site healed well, with only minimal residual scarring. The aesthetic outcomes of the skin-fat composite grafts were outstanding, with a mean (±standard deviation) score of 4.2±0.4. The entire surgical and hospitalization periods were welltolerated, and most patients and parents were highly satisfied with the results.
Case 3 was a 4-year-old girl with a 1.5×3 cm2 congenital melanocytic nevus on the left cheek and upper lip adjacent to the ala-cheek groove (Fig. 1A). After complete excision of the nevus, the defect of the cheek was covered with a locally advanced skin flap, and the remaining defect of the upper lip was reconstructed with a 1.5×1.5 cm2 skin-fat composite graft from the left preauricular area. During the follow-up period of 7 months after the surgery, there was only minimal scarring along the graft boundary and donor site. The color match, texture, and contour of the graft were outstanding, and the aesthetic outcome was rated as a 4.5 (Fig. 1B).
Case 5 was a 5-year-old girl with a 2.5×2.5 cm2 congenital melanocytic nevus on the left ala and lateral nasal wall extending to the cheek (Fig. 2A). This was the largest facial nevus of all our patients. Considering the size of the facial defect, three pieces of skin-fat composite graft from both preauricular areas were used in the reconstruction. Twelve months after the surgery, the color match and texture of the cheek grafts were excellent, and those of the alar graft were acceptable (Fig. 2B). The aesthetic outcome was rated as a 3.75 (Fig. 2C).
Reconstruction of relatively small defects with composite grafts also provided fine results. Case 6, a 16-year-old boy with 1×2 cm2 congenital melanocytic nevus on the right ala, underwent complete excision of the nevus and reconstruction with a skin-fat composite graft (Fig. 3A). The resultant color and texture were outstanding, and the contour was acceptable, with an overall aesthetic outcome rating of 3.75 (Fig. 3B). Case 8, a 3-year-old girl, also had a small 1×1 cm2 congenital melanocytic nevus on the right ala and cheek, and the defect was successfully reconstructed with a skin-fat composite graft after complete excision (Fig. 4A). The outcome was excellent, being rated as a 4.25 (Fig. 4B).
The treatment of CMN must eliminate the risk of melanoma development and recurrence, as well as produce aesthetic and psychological benefits. The method of treatment depends on the size and location of the nevus. Treating medium-sized facial nevi involves particularly careful planning of the reconstruction approach after surgical excision. Coverage of a facial skin defect that cannot be primarily closed must provide an appropriate color match, texture, and contour.
Skin-fat composite grafts are a superb option for reconstructing facial skin defects. Previous studies reported the use of skin-fat composite grafts in facial reconstruction, but they mainly focused on nasal defects in adults [8-11]. These studies reported that skin-fat composite grafts provide a better color match and texture than skin-only grafts for nasal defect reconstruction. The results of our study supported this and were also applicable to other facial parts. Our skin-fat composite grafts produced an outstanding mean aesthetic score of 4.2 points, based on color match, texture, and contour. They did not cause visible scars, tension-induced contour deformities of the surrounding tissues, or dog-ear deformities. Furthermore, the surgical and hospitalization periods were well-tolerated. Creating these grafts is better tolerated than local flaps, especially in children, because the technique involves simpler procedures and shorter operation times.
Another advantage of the skin-fat composite graft is its high survival rate, which is similar to that of skin-only grafts [8]. We covered eleven defects of varying sizes after nevi excision, and not one graft was lost. To increase graft survival, we applied local hyperbaric oxygen to some patients for up to 5 days postoperatively. We created a temporary chamber around the graft, into which we instilled continuous oxygen at 5 L/min. The efficacy of this technique requires further study, but we believe it reduces venous congestion and aids survival.
The donor material for a skin-fat composite graft includes various options. It is important to select a proper donor site that provides good match with the skin surrounding the defect. Previous studies have reported the usefulness of the preauricular area as the donor site in head and neck reconstruction [9,11]. We used the preauricular area on one or both sides of the face as the donor site, depending on the defect. In this manner, we successfully covered facial defects up to 2.8 ×2.5 cm2. Each donor site was primarily closed, and no complications developed. The scars were minimal.
One known limitation of skin-fat composite grafts is their bulkiness compared to skin-only grafts [8]. We controlled the thickness through defatting, and we attained appropriate contours. By meticulous defatting and trimming, complications such as protuberance and volume depletion were avoided. Contraction, the main cause of distortion of marginal tissues with skin grafts, occurred minimally and was barely recognizable.
There was only one patient who received additional surgery for remnant nevus. The remnant nevus was well managed with additional excision and primary closure. No patient required surgical revision for an aesthetic problem or complication.
We conclude from our study that skin-fat composite grafts are suitable for the management of small or medium-sized facial CMN. The technique was applicable to young patients from 3 to 16 years old, which is the usual age range of candidates for treatment of facial CMN. We also found that the aesthetic outcome of skin-fat composite graft was outstanding and not inferior to that of local flaps. Applying skin-fat composite grafts for the management of medium-sized facial CMN is an excellent technique that fully treats the lesion and provides superb color, texture, and contour, while minimizing the morbidities at donor sites and surrounding tissues.
Fig. 1.
Case 3. (A) Preoperative view of a 4-year-old girl with a congenital melanocytic nevus on the ala-cheek groove, cheek, and upper lip. After excision of the nevus, a skin-fat composite graft from the left preauricular area was used to reconstruct the defect. (B) Postoperative view at 7 months after surgery.
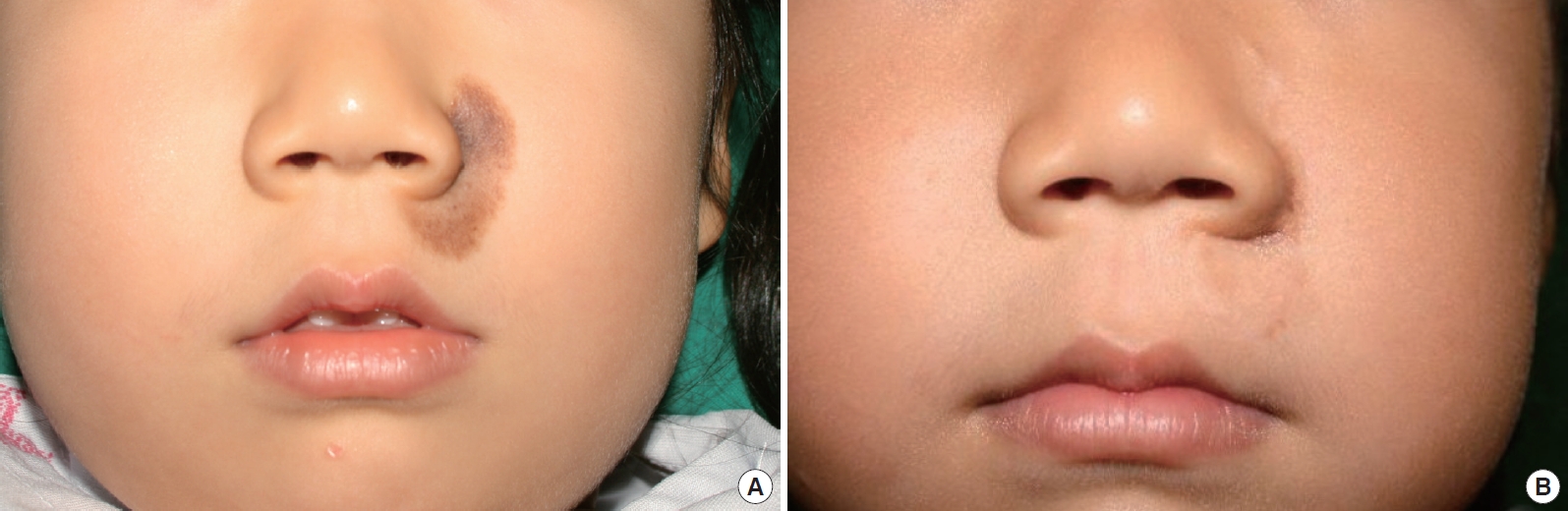
Fig. 2.
Case 5. (A) Preoperative view of a 5-year-old girl with acongenital melanocytic nevus on the nose and cheek. (B) After excision of the nevus, skin-fat composite grafts from the preauricular areas on both sides were used to reconstruct the defect. (C) Postoperative view at 12 months after surgery.
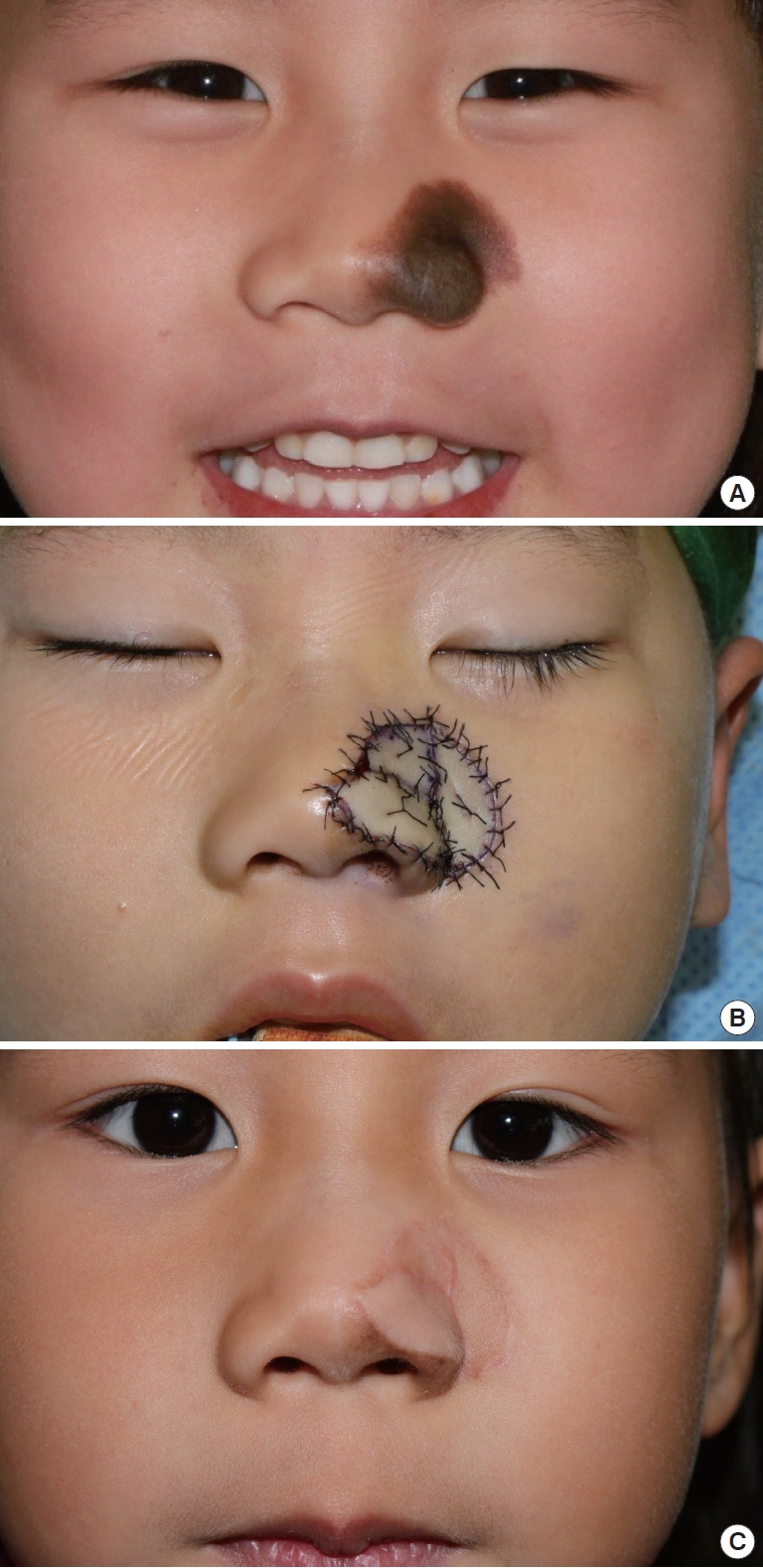
Fig. 3.
Case 6. (A) Preoperative view of a 16-year-old boy with a congenital melanocytic nevus on the right ala. After excision of the nevus, a skin-fat composite graft from the right preauricular area was used to reconstruct the defect. (B) Postoperative view at 16 months after surgery.
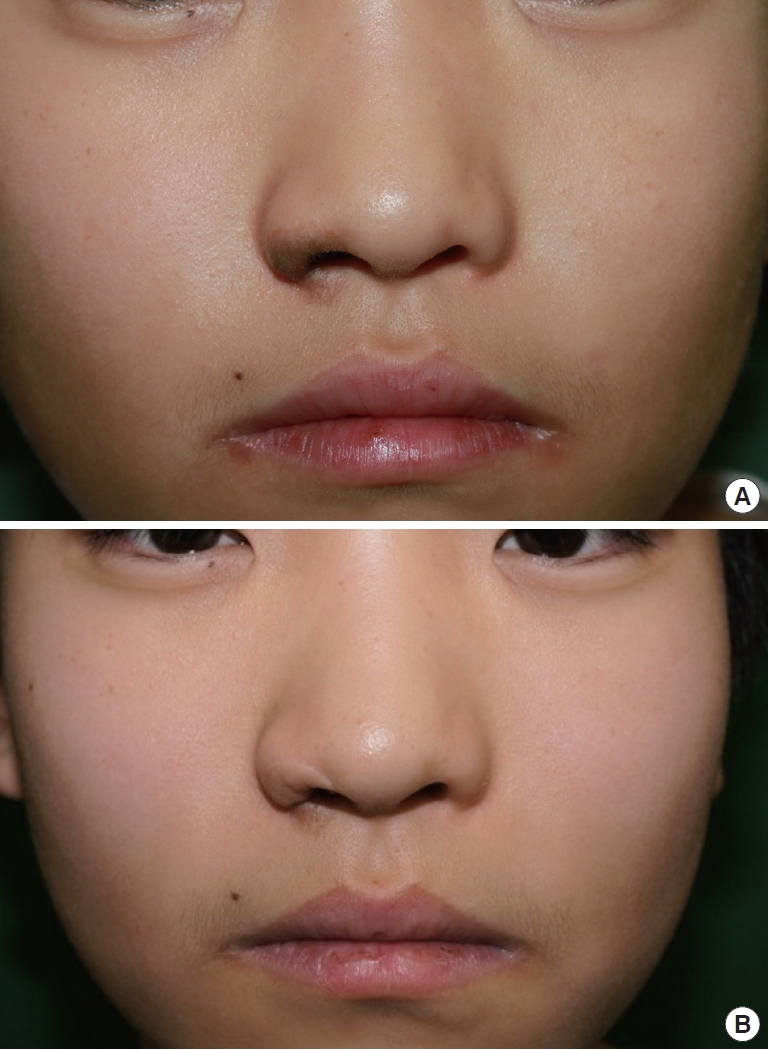
Fig. 4.
Case 8. (A) Preoperative view of a 3-year-old girl with a congenital melanocytic nevus on the ala and cheek. After excision of the nevus, a skin-fat composite graft from the right preauricular area was used to reconstruct the defect. (B) Postoperative view at 24 months after surgery.
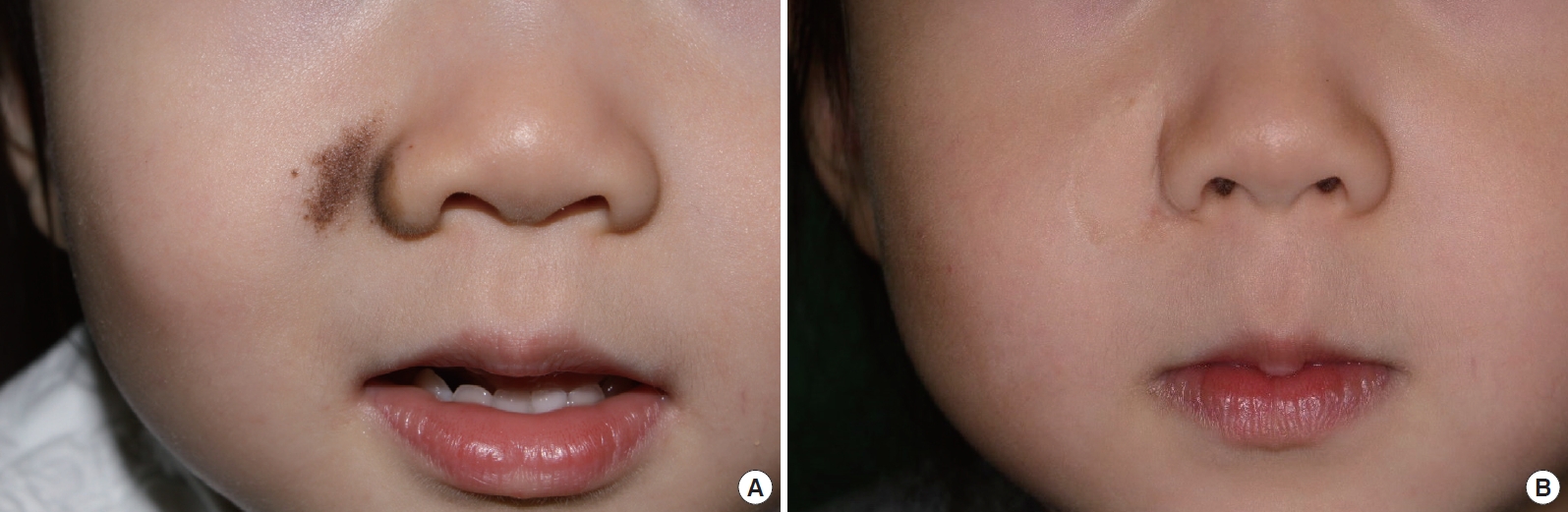
Table 1.
Patient and nevus characteristics and aesthetic outcomes
| Case No. | Sex | Age (year) | Location | Width (cm) | Length (cm) | Follow-up (months) | Aesthetic outcomea) |
|---|---|---|---|---|---|---|---|
| 1 | F | 8 | Upper lip | 2 | 2.5 | 12 | 4.75 |
| 2 | F | 11 | Oral commissure | 1.2 | 2 | 8 | 4 |
| 3 | F | 4 | Ala-cheek groove and upper lip | 1.5 | 3 | 7 | 4.5 |
| 4 | M | 6 | Cheek | 1.5 | 2.5 | 25 | 4.25 |
| 5 | F | 5 | Nose and cheek | 2.5 | 2.5 | 12 | 3.75 |
| 6 | M | 16 | Ala | 1 | 2 | 16 | 3.75 |
| 7 | M | 7 | Lower eyelid | 0.9 | 1.4 | 6 | 3.75 |
| 8 | F | 3 | Ala and cheek | 1 | 1 | 32 | 4.25 |
| 9 | F | 16 | Lower eyelid | 0.5 | 1 | 6 | 3.75 |
| 10 | M | 4 | Columella and ala | 1 | 2 | 8 | 4.5 |
| 11 | F | 3 | Upper eyelid | 1 | 1.2 | 13 | 4.5 |
| Meanb) | 7.6 ± 4.6 | 1.3 ± 0.5 | 1.9 ± 0.7 | 13.2 ± 8.0 | 4.2 ± 0.4 |
A total of 11 patients were enrolled. The mean age was 7.6 years old and the mean follow-up period was 13.2 months. All the defects originated from nevi excision were reconstructed with skin-fat composite grafts harvested from preauricular areas. Two patients (case 1, case 5) required multiple grafts from bilateral preauricular areas.
REFERENCES
1. Rhodes AR, Wood WC, Sober AJ, et al. Nonepidermal origin of malignant melanoma associated with a giant congenital nevocellular nevus. Plast Reconstr Surg 1981;67:782-90.


2. Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr 2012;161:240-5.


3. Castilla EE, da Graca Dutra M, Orioli-Parreiras IM. Epidemiology of congenital pigmented naevi: I. Incidence rates and relative frequencies. Br J Dermatol 1981;104:307-15.


4. Tannous ZS, Mihm MC Jr, Sober AJ, et al. Congenital melanocytic nevi: clinical and histopathologic features, risk of melanoma, and clinical management. J Am Acad Dermatol 2005;52:197-203.


5. Pearson GD, Goodman M, Sadove AM. Congenital nevus: the Indiana University’s approach to treatment. J Craniofac Surg 2005;16:915-20.


6. Viana AC, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An Bras Dermatol 2013;88:863-78.




7. Olazagasti J, Kleinerman R, Eisen DB. Composite full-thickness skin and fat graft for nasal reconstruction. Dermatol Surg 2014;40:337-40.


8. Gurunluoglu R, Shafighi M, Gardetto A, et al. Composite skin grafts for basal cell carcinoma defects of the nose. Aesthetic Plast Surg 2003;27:286-92.


9. Kreutzer C, von Gregory HF, Fischer H. Skin-fat-graft: a simple tool for reconstruction of small deep defects of the nose. Facial Plast Surg 2014;30:247-59.









